Supporting materials
Download
Download this article as a PDF

Simulate a neuron in the classroom.
The nervous system is not only fascinating but also probably one of the most complex topics in school biology lessons, particularly because working with real neurons is not feasible at school. In this article, we describe an activity that uses a cellophane membrane to explore how the resting potential is generated in a neuron. Suitable for students aged 16–19, the activity takes approximately 90 minutes.
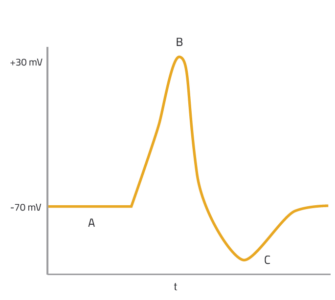
To transfer information, neurons need to be able to generate and maintain a membrane potential: a voltage difference between the intracellular and extracellular media that is focused along the cell membrane. The voltage difference in an unexcited neuron is referred to as the resting potential. Stimulating this neuron can alter the resting potential, causing an action potential: the electrical impulse by which the neuron transmits information. Before the neuron can fire again, the resting potential needs to be re-established (figure 1). But how is the resting potential generated and maintained? The answer lies partly with the semi-permeable nature of the cell membrane.
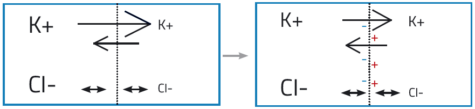
Among other constituents of the intracellular and extracellular media are dissolved ions including sodium (Na+), chloride (Cl–), organic anions (A–) and, most importantly, potassium (K+). Once a neuron has fired and the resting potential begins to be re-established, the concentration of K+ ions is higher inside the neuron than outside. Unlike most other ions, K+ ions can pass freely into and out of the cell, via specialised ion channels in the membrane. Driven by the concentration gradient, K+ ions diffuse out of the neuron, causing a net movement of positive charge (figure 2). This causes a voltage difference across the membrane, with the intracellular medium being more negatively charged than the extracellular medium. This is the resting potential, with a value of around –70 mV.
Although there are additional factors involved in establishing the resting potential in a neuron, the combined contribution of the concentration gradient and the electrical properties of anions and cations can easily be demonstrated in the classroom using cellophane as a semi-permeable membrane, as described below.
Before the activity, it is useful to cover the basic principles of diffusion and cell membranes with your students. Instructions for hands-on activities involving the properties of the cell membrane and diffusion through membranes can be downloaded from the additional material sectionw1.
For each group of 2–4 students, you will need:
Before beginning the activity, discuss with your students how they think a voltage difference can occur in the cell, and which cell components are important in establishing it. Briefly introduce the resting potential. Then, ask your students to:
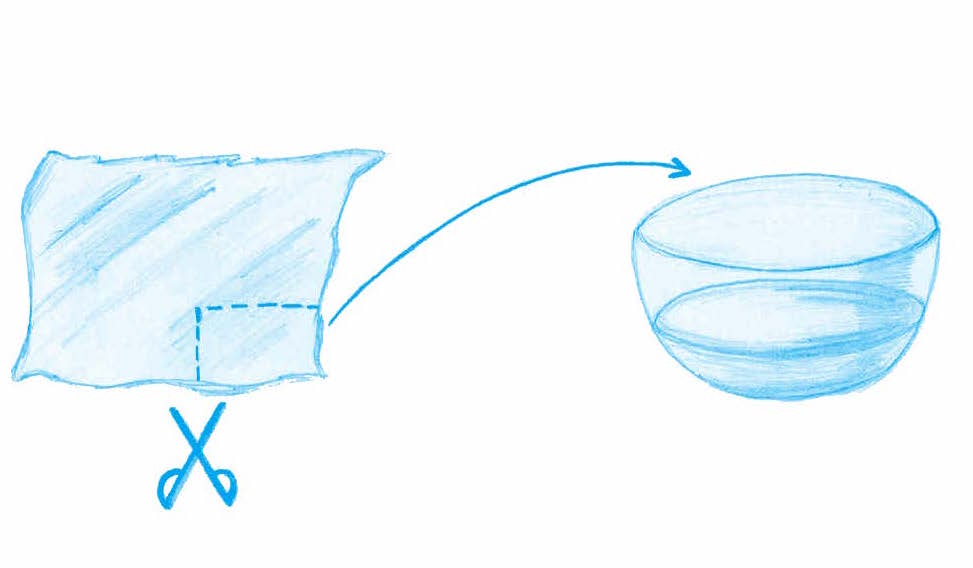
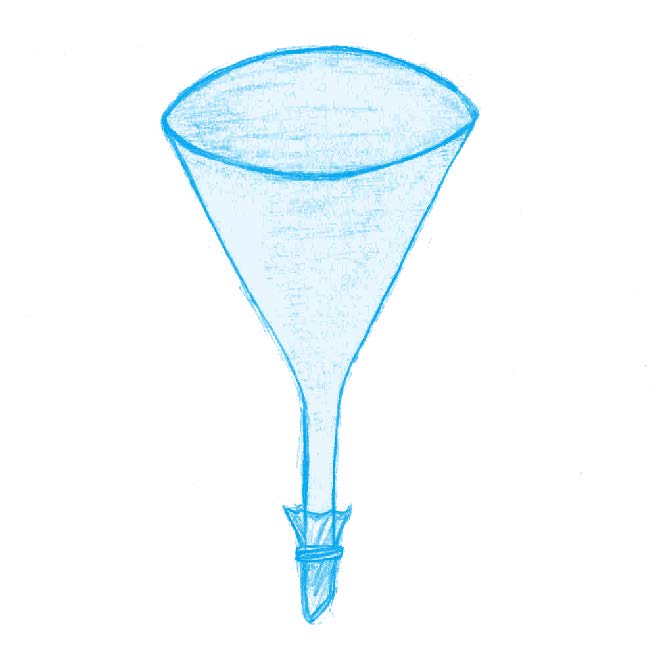
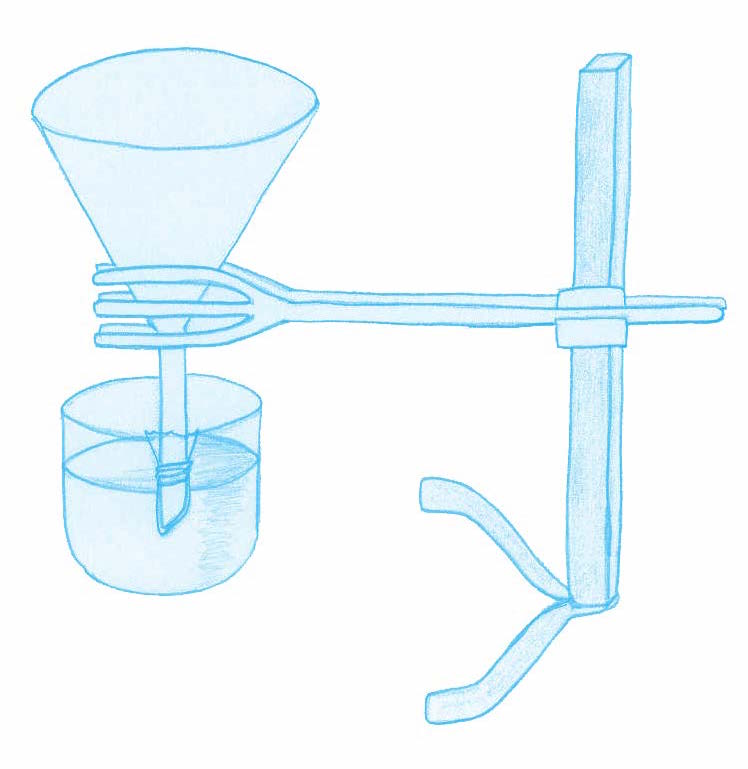
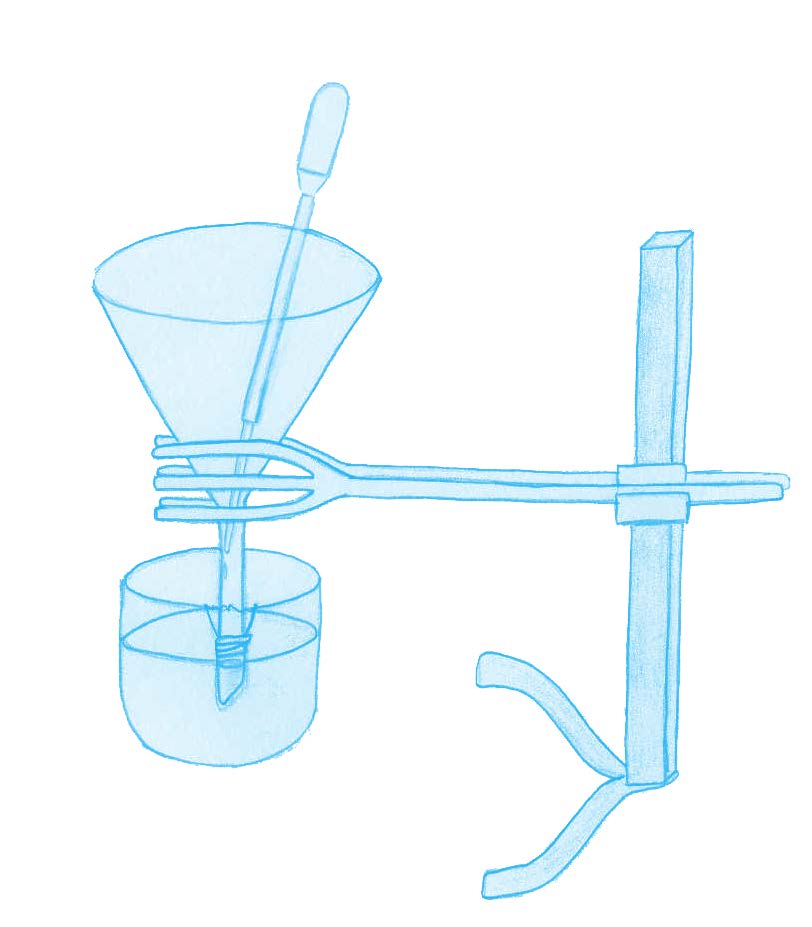
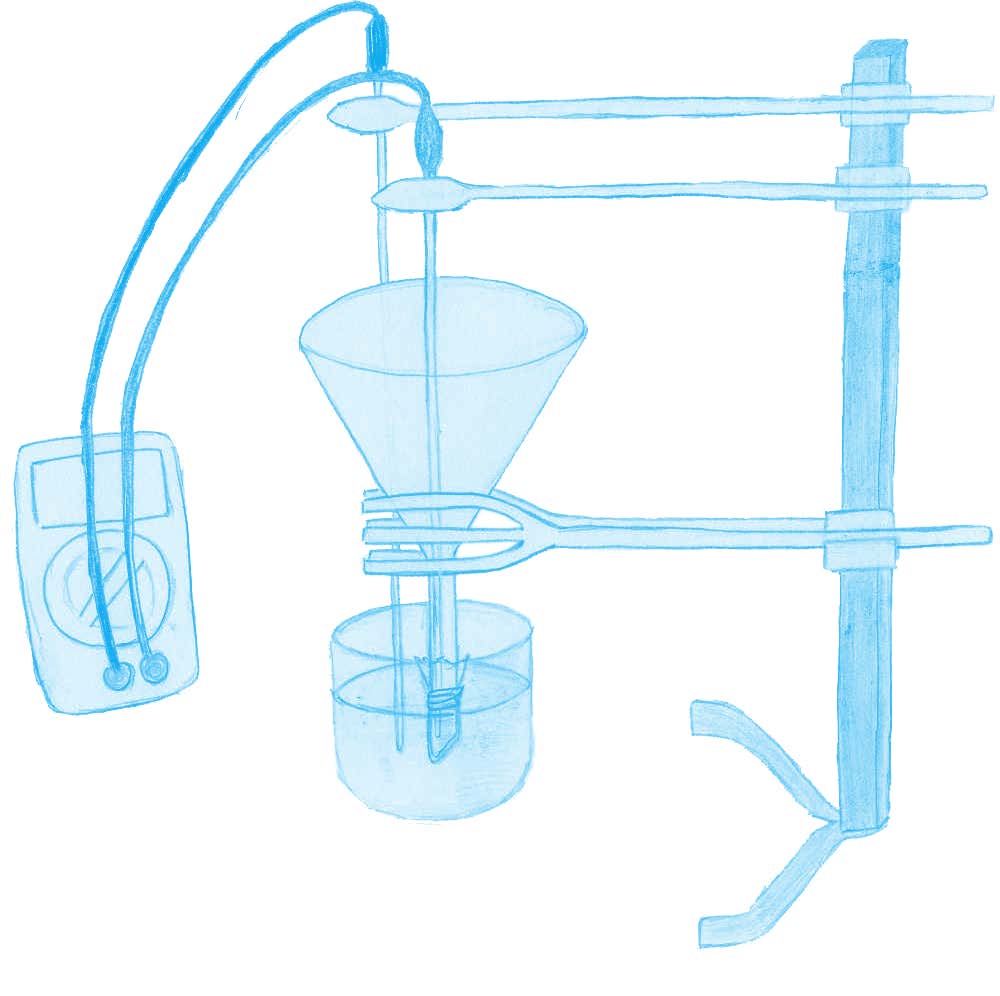
Ask your students:
As in the real-life neuron, this experiment relies on two components: a concentration gradient and the semi-permeable properties of the cellophane wrapping. Like the membrane of a neuron, the cellophane is permeable to K+ ions but almost non-permeable to Cl– ions. As a result, as in the neuron, there is a gradual, net diffusion of K+ ions out of the funnel (0.1 M KCl) and into the glass bowl (0.01 M KCl). If the electrodes are placed carefully, without puncturing the cellophane, the voltage of the solution in the funnel can be seen to become more negative. The initial setting of 200 mV on the voltmeter is arbitrary, to ensure that the final reading is similar to that of the real-life resting potential.
While realistic, this experiment is not a complete model of how the resting potential is established and maintained. In a neuron, the extracellular and intercellular media contain more than just K+ and Cl– ions, and there are additional mechanisms that determine the membrane’s permeability. However, this activity offers an opportunity to discuss the accuracy of the model, and to introduce other aspects of neurobiology such as ions channels, the sodium–potassium pump and the action potential.
Alternatively, you could ask your students to discuss hypothetical scenarios, for example, using additional solutions, a membrane with different properties or different concentrations of KCl.
Simple models can be very helpful for understanding complex processes that take place in nature. This article describes a practical activity to unravel how neurons work. All the materials required can easily be obtained and the instructions are easy to follow, making the experiment suitable for students to perform in groups.
The activities could be used to combine different topics in biology, chemistry and physics.
Those interested in deepening their knowledge about the subject can also find complementary teaching activities in the web references section.
Mireia Güell Serra, Spain
Download this article as a PDF