From Petri dish to plate: the journey of cultivated meat Understand article
Future food: would you bite into a test-tube burger or a Petri dish steak? How do we make lab-grown meat, and what might it mean for health, farming, and the environment?
In 2013 a scientist sat with two food experts, taking turns biting into a hamburger on TV. “Close to meat”, “not as juicy”, “a mouthfeel like meat”, declared the tasters.[1] Although that hamburger may not have won any culinary awards, it certainly marked a milestone in our culinary history. This verdict described the world’s first ever lab-grown burger.[2] It cost around $330 000 and took two years to make. Ten years on, the reality of having lab-based food on our dining tables may be a tiny bit closer.

Image: PA Images / Alamy Stock Photo
Emissions from livestock account for 14.5% of our total greenhouse gas emissions, mostly due to methane emissions from ruminant animals.[3] Livestock production is linked to a loss of biodiversity and requires a lot of land and resources, including water.[4] Furthermore, industrial animal farming raises additional ethical concerns about animal welfare. Animal-free meat alternatives, mostly based on plant protein sources like tofu or pulses, are on the rise to cater to an environmentally conscious population. In contrast, cell-based/cultured meat aims to replicate meat exactly by cultivating animal cells in a lab rather than raising animals on a farm.
Culturing cells outside an organism has been routine in research labs for decades. Cell cultures are key for testing hypotheses in their initial stages before they are explored in whole-animal models. Large-scale production of vaccines, like the polio and chicken pox vaccines, is also achieved with cell culture.[5] However, cellular agriculture for food production is a much more recent aspiration and, if achieved, we could be eating lab-grown beef, chicken, or seafood in the near future. In fact, you can already munch on lab-grown chicken tenders in Singapore, where Eat Just’s cultured meat got regulatory approval in 2020.[6]
So, how does one go about making a burger in the lab?
Making meat in the lab
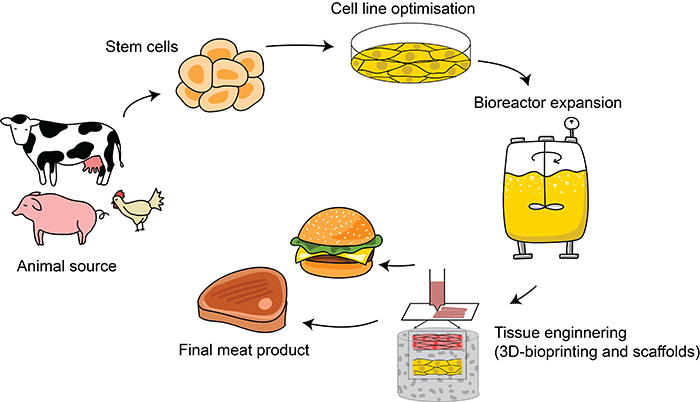
In a nutshell, cultured meat production involves collecting the right animal cells, optimizing them for growth outside of the animal, scaling up the production of these animal cells (along with tissue engineering to mimic animal tissue), and finally processing them into a food product (figure 2).
The first question is, which are the right cells? Meat is mostly muscle, with protein-rich cells arranged in fibre bundles. These muscles and the adjoining fat tissue are fed by a network of blood vessels. So, the ideal starting material would be cells that are able to replicate in large numbers and generate these meat tissue components.[8]
Which cells to use?
Adult stem cells from muscle tissue are a common choice for cultivated meat. They can be acquired by taking tissue samples (biopsies) from a live animal or tissue left from a slaughtered animal (figure 3). However, these cells can only replicate for a short period in culture, so a constant supply of new cells is needed.[9] Researchers are trying to engineer adult stem cells with improved capacity to self-renew, but this remains challenging, so some labs are exploring the potential of pluripotent stem cells (PSCs), specifically embryonic stem cells (ESCs)[10] or induced pluripotent stem cells (iPSCs) made by engineering mature adult cells (figure 3).[11,12] These can replicate indefinitely and give rise to all cell types. However, guiding them to differentiate into the cell type you want is challenging.
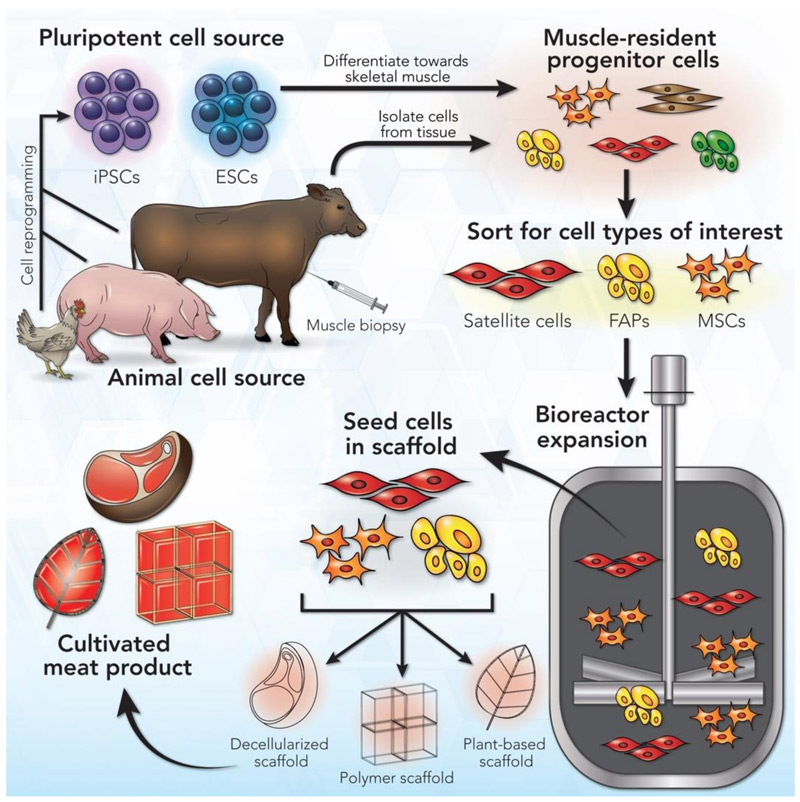
Image from Ref. [8], CC BY 4.0
Scaling up
Cell culture in the lab usually happens in flat dishes no larger than a Frisbee (figure 4), but producing cells for commercial food production requires a massive scale. Large bioreactors that can hold thousands of litres of culture must provide optimal sterile conditions for cell growth, such as the correct temperature, CO2 levels, nutrient circulation, and any scaffolding needed.

Image courtesy of the author
The cells are grown in a liquid medium rich in nutrients like amino acids, glucose, vitamins, and salts.[5] One common component is fetal bovine serum (FBS), which contains many growth factors that can maintain cell proliferation. FBS is harvested from the fetuses of pregnant cows after slaughter, so its use is controversial. It is also expensive and variable from batch to batch, so it presents a significant hurdle to making cellular meat economically and ethically sustainable. Supplementation with protein-rich extracts from plants or microorganisms, like yeast and cyanobacteria, along with protein growth factors produced in the lab, can be used instead, but it is hard to achieve optimal cell growth with these alternatives. However, many companies are investigating these cheaper options and some have already announced a move away from the use of FBS, which is a promising move.[13]
Another significant challenge is making different cuts of meat. An unstructured mass of cells won’t have the right texture. Large-scale liquid cell culture makes it easier to produce ground meat rather than structured meat like a steak. Tissue engineering combined with 3D bioprinting are now being optimized to grow muscle, fat, and blood capillary fibres on special scaffolding matrices ( made from, e.g., collagen) to form them into the appropriate shape and texture (figure 3).[14] Two Israeli companies unveiled lab-grown steaks in 2021 and 2018.[15,16] It will likely be a while before these products are commercially available because scaling up production with edible and cheap scaffolds is no easy feat.
Comparisons with conventional meat
How does the environmental and health impact of cultured meat compare with conventional meat?
A 2020 study compared the greenhouse gas (GHG) emissions of several nutrition sources, and found that beef has the highest GHG emissions (median of about 25.6 kg CO2 equivalents), far ahead of poultry meat (around 3.35 kg CO2 equivalents) or pig meat (around 5.76 kg CO2 equivalents). In comparison, the emissions from cultured meat (median of about 5.56 CO2 equivalents) were five times lower than that of farmed beef but comparable to or higher than poultry and pig meat. Given that it is a fast-evolving new industry, emissions projections for cultured meat are quite variable, with different assumptions made about the scale and efficiency of production, but a large fraction of the emissions for cultured meat comes from its energy use. A shift towards more renewable energy sources in the future and a standardized energy-efficient production could potentially reduce the carbon footprint. Currently, the use of plant-based foods is by a large margin the most sustainable protein source, with carbon emissions of tofu, peas, and pulses being 4–17 times lower than the lowest emitting animal protein source.[4] In the absence of a significant difference in carbon emissions, farmed versus cultured poultry might be a question of animal welfare rather than an environmental choice.
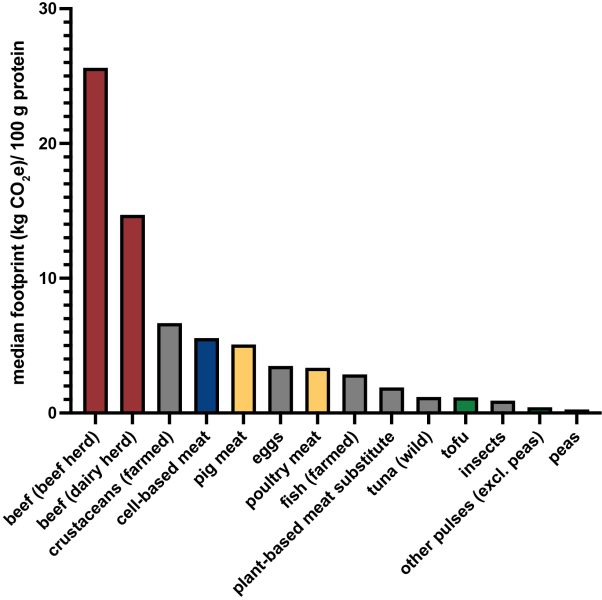
Image replotted from data in Ref. [4].
The sterile conditions used in cell culture reduce the risk of spreading zoonotic diseases, which can jump from animals to humans. However, the need for sterility is an additional challenge. The current setup for cultured meat builds on the pharmaceutical-grade production setup for therapeutics, which requires aseptic techniques (including the use of single-use plastics) for handling the cells and for manufacturing the growth media, which increases the environmental costs.
The nutritional value of cultured meat can vary depending on the type and growth conditions of the cells used to make it. Many cultured meat companies aim to mimic the taste and nutritional profile of farmed meat exactly. However, some labs are exploring ways to improve the nutritional content of the meat, for example, by using cells engineered with increased omega-3 fatty acids or reduced cholesterol to make healthier meat.
Although the cost of producing cultured meat is now much lower than for the initial prototypes, it remains higher than for conventional meat and this is a barrier to its introduction as a replacement for conventional meat. Eat Just’s cultured chicken costs around $23 in restaurants, which is comparable to premium-quality meat. The high cost is mostly driven by the costs of cell growth (mainly the growth medium), so further scale-up and more efficient production methods could substantially reduce the costs of cultured meat.
Where do we go from here?
Technical aspects aside, the concept of cultured meat itself raises bigger questions. Cultured meat aims to convert those who still crave the taste of meat, eat it frequently, and are not big fans of plant-based meat. It offers a more sustainable option without having to give up eating meat. Cellular agriculture also opens a whimsical but ethically questionable door to cultivating exotic animal meat, like zebra or tiger (a UK company is already working on this). Marketed as ‘Innocent meat’, ‘Good meat’, ‘Clean meat’, it is possible that cultured meat would find new consumers in vegetarians or vegans who gave up meat because of ethical reasons, which would not necessarily represent an environmental gain. However, the use of animal cells in cultured meat could still be a dividing factor. There are also open questions about the need for cultured meat. A plant-based diet remains the least energy-intensive and most sustainable source of protein, and even a shift to poultry and pork from beef would reduce our environmental impact. However, food can have complex emotional and cultural ties, and getting most of the world to shift to more sustainable food habits is crucial for our collective future. Whichever way your taste buds and values lie, this new technology could potentially provide additional options for living in a way that is kinder to the planet and the animals we share it with.
References
[1] An article in The Guardian about food experts trying the first synthetic hamburgers: https://www.theguardian.com/science/2013/aug/05/world-first-synthetic-hamburger-mouth-feel.
[2] Post MJ (2014) Cultured beef: medical technology to produce food. J Sci Food Agric 94: 1039–1041, doi:10.1002/jsfa.6474
[3] Gerber PJ et al. (2013) Tackling climate change through livestock : a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome.
[4] Santo RE et al. (2020) Considering Plant-Based Meat Substitutes and Cell-Based Meats: A Public Health and Food Systems Perspective. Frontiers in Sustainable Food Systems 4. doi:10.3389/fsufs.2020.00134
[5] Segeritz CP, Vallier L (2017) Basic Science Methods for Clinical. Academic Press. ISBN: 9780128030776
[6] The Reuters article about Singapore approving the sale of lab-grown meat: https://www.reuters.com/article/eat-just-singapore-idINL4N2II0BV.
[7] Lu H et al. (2022) Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review. Bioengineering 9: 787.
[8] Reiss J, Robertson S, Suzuki M (2021) Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. International Journal of Molecular Sciences 22: 7513. doi: 10.3390/ijms22147513
[9] Redondo PA et al. (2017) Elements of the niche for adult stem cell expansion. J Tissue Eng 8: 1–18. doi: 10.1177/2041731417725464.
[10] Kinoshita M et al. (2021) Pluripotent stem cells related to embryonic disc exhibit common self-renewal requirements in diverse livestock species. Development 148: dev.199901. doi: 10.1242/dev.199901.
[11] Verma R, Lee Y, Salamone DF (2022) iPSC Technology: An Innovative Tool for Developing Clean Meat, Livestock, and Frozen Ark. Animals 12: 3187. doi: org/10.3390/ani12223187
[12] Su Y et al. (2021) Establishment of Bovine-Induced Pluripotent Stem Cells. Int J Mol Sci 22: 10489. doi:10.3390/ijms221910489
[13] Messmer T et al. (2022) A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nature Food 3: 74–85, doi:10.1038/s43016-021-00419-1.
[14] Kang DH et al. (2021) Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat Commun 12: 5059. doi:10.1038/s41467-021-25236-9 (2021).
[15] An article in The Guardian about a steak 3D printed with bovine stem cells: https://www.theguardian.com/environment/2021/dec/08/worlds-largest-lab-grown-steak-unveiled-by-israeli-firm.
[16] An article in The Guardian on the first lab-grown steak: https://www.theguardian.com/environment/2018/dec/14/worlds-first-lab-grown-beef-steak-revealed-but-the-taste-needs-work.
Resources
- Watch a video about the hidden costs (social, economic and environmental) of a vegan diet on the BBC.
- Learn about cultivated meat cell lines.
- Read an article on the cultural and social aspects of cultivated mean: Bryant CJ (2020) Culture, meat, and cultured meat. J Anim Sci 98: skaa172. doi: 10.1093/jas/skaa172
- Which proteins have the lowest-carbon protein? Read this BBC Future article to find out.
- Teach about the science of honeybees and their sugary product through a series of hands-on activities: Scheuber T (2023) To bee or not to bee: the biology of bees and the biochemistry of honey. Science in School 62.
- Learn about the toxicology and the physiological effects of drugs by using Daphnia as a model organism: Faria HM, Fonseca AP (2022) From drugs to climate change: hands-on experiments with Daphnia as a model organism. Science in School 59.
- Explore chemotaxis and the scientific method with slime mould: Buchta A, Dunthorn M (2023) Moving slime: exploring chemotaxis with slime mould. Science in School 62.
- Investigate antibiotic resistance and drug development: Fernandez MD, Soler ML, Godinho T (2021) Microbiology: Discovering antibacterial agents. Science in School 55.
- Learn about the importance of animal use in research and some cutting-edge approaches to minimizing it: Schmerbeck S et al. (2021) Organ-on-chip systems and the 3Rs. Science in School 54.
- Find out about plant disease and pathogens: Harant A, Pai H, Cerfonteyn (2023) Plant pathology: plants can get sick too! Science in School 62.
- Read an article about the environmental effects of food packaging: Barlow C (2022) Plastic food packaging: simply awful, or is it more complicated? Science in School 56.
- Learn about the renewable energy transition in Europe: Süsser D (2023) Clean energy for all: can sun and wind power our lives? Science in School 62.