Supporting materials
Download
Download this article as a PDF

How do social drugs affect metabolism? How is toxicity measured? How does climate change affect water ecosystems? Promote active learning by investigating these questions with Daphnia.
Daphnia, a freshwater microcrustacean keystone species, is a model organism with many practical advantages: simplicity of culture under laboratory conditions, small body size (2–5 mm), short life cycle, easy handling, high fecundity, parthenogenetic reproduction, and low maintenance costs.[1,2] Due to their translucent chitinous exoskeleton, it is possible to observe Daphnia’s internal organs under a microscope or binocular magnifying glasses.[3] Since they are very sensitive animals, and because they have fundamental biological responses very similar to those of humans, they are used as models to test the effects of various ‘social drugs’ (also called recreational drugs), such as coffee, tobacco, and alcohol, and are used to evaluate water quality.[2] Freshwater ecosystems, which are among the most threatened in the world, provide drinking and irrigation water, food, climate regulation, erosion prevention, and recreation for human society. Climate change is increasing the salt concentration of freshwater, with drastic effects on the health and survival of freshwater organisms.
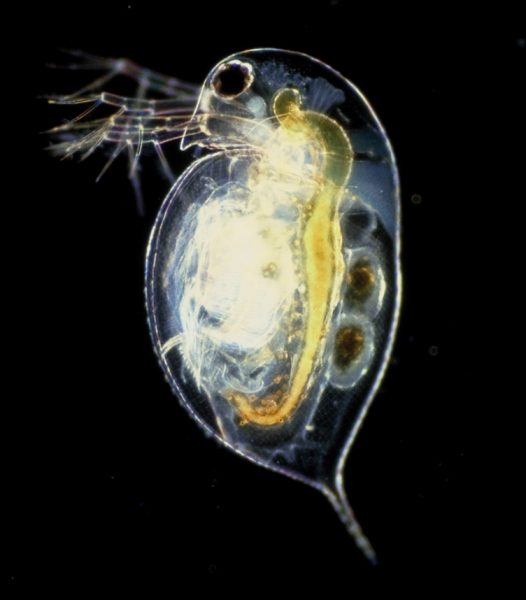
The hands-on activities outlined in this article are suitable for students aged 14–19 and are a valuable way to evaluate the effects of social drugs on the cardiovascular system and the influence of salinization on Daphnia survival. By investigating these effects, students learn to record and manage experimental data to draw their own conclusions. From the conclusions, students can consider the implications for human health, and understand the effects of climate change on freshwater ecosystems.
Cultures can be maintained in containers with a maximum of 30 Daphnia per litre in chlorine-free water with a pH of 7–8, at 18–25°C, over a controlled photoperiod of 16 h and fed 2–3 times per week with microalgae or yeast (5 drops/litre). Low levels of dissolved oxygen are lethal.
To prepare a yeast (unicellular fungus) suspension, add chlorine-free water to a 1 litre container and stir in 2 g of baker’s yeast. Store the suspension in a refrigerator (4°C) and always agitate before use to resuspend the yeast. Add 5 drops/litre of this food to Daphnia cultures 2–3 times per week.
Microalgae are microscopic photosynthetic organisms. They play a central role in life on Earth, as they form the basis of the food web in many aquatic ecosystems. Scenedesmus is a nonmotile green microalgae, typical of freshwater phytoplankton, that is easy to culture and maintain in the laboratory and is routinely used to feed Daphnia.
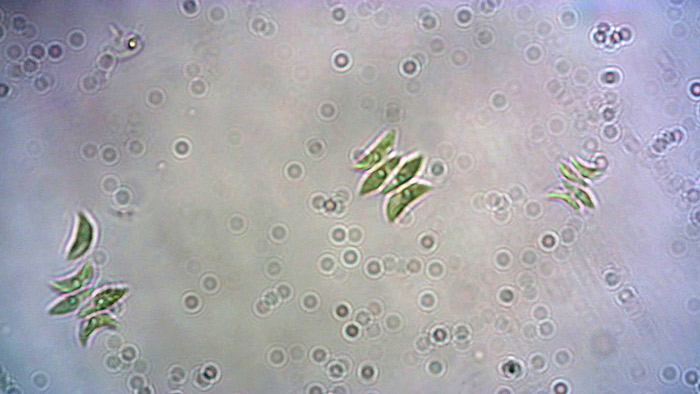
Cultures of Scenedesmus require the use of chlorine-free water with a pH of 7–8, at a minimum temperature of 16°C (18 to 25°C), and with a minimum photoperiod of 12 h provided by an LED (or fluorescent) aquarium light. The microalgae culture should be kept in an aerated container (bioreactor) to provide the CO2 required for photosynthesis and prevent sedimentation of microalgae in the bottom of the vessel, which greatly limits their reproduction. Every 15 days, the culture should be supplemented with mineral salts from 3–5 drops/litre of commercial liquid plant fertilizer.

Social-drug consumption causes changes to the central nervous system, resulting in physiological and/or behavioural changes. Stimulant drugs (caffeine, nicotine) are characterized by increasing body metabolism, whereas depressor drugs (alcohol) decrease it. The heart rate can be monitored as an optimal indicator of these changes.
Observing how drugs affect a living organism in real time allows students to establish a relationship with the possible effects of drugs in their body. The transparent body of Daphnia allows measurement of different physiological parameters using noninvasive optical methods. A bioassay is a procedure that uses living organisms to determine the effects of a chemical. The activity requires around 2 hour to complete but can be done in 1 h if the solutions are prepared beforehand.
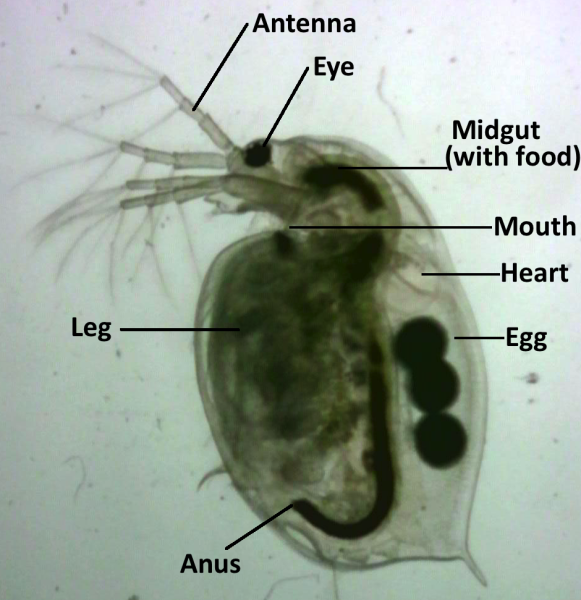
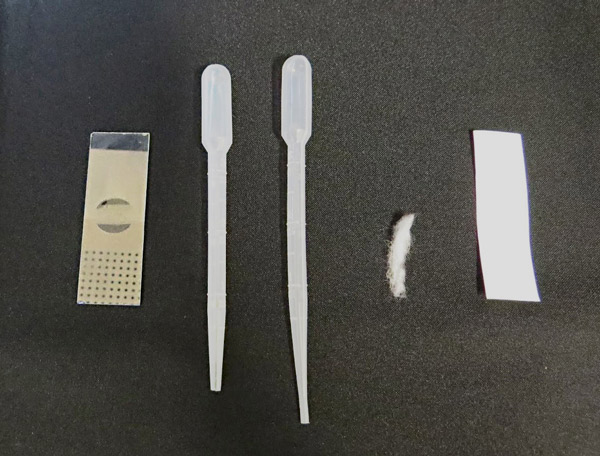
On the provided worksheet (see sample table), students predict the effects of the social drugs on the heart rate of Daphnia. Before starting experimental work, watch this video to learn how to find the Daphnia heart and how to count heartbeats.
Caution: if excess cotton is used, it may be difficult to observe the Daphnia heart.
| Prediction (according to key below) | Control heart rate (bpm) | Experimental heart rate (bpm) | |
| Tobacco | |||
| Alcohol (6%) | |||
| Alcohol (12%) | |||
| Alcohol (20%) | |||
| Coffee |
Key:
| +++ | big increase in heart rate |
| ++ | increase in heart rate |
| + | small increase in heart rate |
| 0 | no change in heart rate |
| – | small decrease in heart rate |
| — | small decrease in heart rate |
| — | small decrease in heart rate |
| x | heart stops |
Control counts below 200 beats per minute (bpm) or above 300 bpm should be repeated.
Care must be taken to ensure that experimental solutions do not come into contact with students’ skin, eyes, or mouth.
At the end of the activity, students must wash their hands.
The transparent bodies of Daphnia allows visual observation of internal organs and measurement of physiological characteristics, such as heart rate. They can be used as a model system for toxicology because they are highly sensitive to environmental disturbances and exhibit rapid physiological responses when exposed to water contamination.[5]
The use of live animals, as part of authentic research experiences, has been shown to increase understanding of scientific concepts. After the experiment, ask students the following questions to promote their understanding of the influence of social drugs on the nervous system using Daphnia as a model organism:
Discuss how Daphnia is a suitable biological model for studying multiple stressors.
Salinization of freshwater ecosystems is an increasing problem due to human activities such as irrigation, resource extraction, accelerated weathering of rocks by acid rain, use of agricultural fertilizers and road salts, mining, and urban construction. This is further exacerbated by climate change due to decreased rainfall, increased evaporation, and an increased need for irrigation.[6,7] The use of bioassay investigations can develop students’ understanding of the effects of salinization on Daphnia survival, thus promoting a conceptual connection between the survival of simple organisms and the effects on freshwater ecosystems. These organisms need to maintain an osmotic balance between the ion concentration within their cells and their body fluids, and this is strongly influenced by the salinity of the surrounding water.
In this activity, students determine the toxicity of NaCl for Daphnia by calculating the LC50 (lethal concentration, 50%) value, which is defined as the toxicant concentration that kills 50% of the exposed organisms. This experiment should take around 20 min.[1]

In the control, the mortality cannot be higher than 10%.
| NaCl (%) | Number of Daphnia that died after | % mortality (after 20 min) | Observations of Daphnia behaviour | ||
| 5 min | 10 min | 20 min | |||
| 0 | |||||
| 0.5 | |||||
| 1 | |||||
| 1.5 | |||||
| 2 | |||||
Do not use detergent to clean the glass material. Detergent residues may be toxic to Daphnia.
After the experiment, discuss the results to promote an understanding of the influence of climate change on the behaviour and survival of Daphnia and other aquatic organisms.[8,9] Questions could include the following:
This activity could also be the basis for a discussion on the ethics of using live animals in research. The use of very simple organisms like Daphnia is fairly uncontroversial, but where would students draw the line? More information on why the use of animals in research can be important and how we can minimise it can be found in this article.
[1] Cahill K (2006) Bioassay investigations with Daphnia. My Environment, My Health, My Choices. University of Rochester.
[2] OECD (2004) Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. doi: doi.org/10.1787/9789264069947-en
[3] Tkaczyk A et al. (2021) Daphnia magna model in the toxicity assessment of pharmaceuticals: a review. Science of the Total Environment 763: 143038. doi: 10.1016/j.scitotenv.2020.143038
[4] Gewin V (2005) Functional Genomics Thickens the Biological Plot. PLOS Biology 3: e219. doi:10.1371/journal.pbio.0030219
[5] Gleichsner AM, Butler SR, Searle CL (2019) Dynamic Daphnia: an inquiry-based research experience in ecology that teaches the scientific process to first-year biologists. CourseSource. doi: 10.24918/cs.2019.2
[6] Kaushal SS et al. (2021) Freshwater salinization syndrome: from emerging global problem to managing risks. Biogeochemistry 154: 255–292. doi: 10.1007/s10533-021-00784-w
[7] Jeppesen E et al. (2020) Salinization increase due to climate change will have substantial negative effects on inland waters: a call for multifaceted research at the local and global scale. The Innovation 1: 100030. doi: 10.1016/j.xinn.2020.100030
[8] Martinez D et al. (2022) Daphnia magna and mixture toxicity with nanomaterials – current status and perspectives in data-driven risk prediction. nanotoday 43: 101430. doi: 10.1016/j.nantod.2022.101430
[9] Müller M et al. (2018) Temperature-driven response reversibility and short-term quasi-acclimation of Daphnia magna. PLOS ONE 13: e0209705. doi: 10.1371/journal.pone.0209705
Download this article as a PDF