Supporting materials
AFM instructions (Word document)
Download
Download this article as a PDF

Would it not be fascinating to observe and manipulate individual molecules? Patrick Theer and Marlene Rau from the European Molecular Biology Laboratory explain how, with an atomic force microscope, you can do just this. You could even build your own.
The idea of looking at single molecules or atoms has fascinated scientists for over a hundred years. This ambitious goal was first achieved in 1981 with the invention of scanning tunnelling microscopy, for which Gerd Binnig and Heinrich Rohrer at the IBM Research Laboratory in Rüschlikon, Switzerland, were awarded the Nobel Prize in Physics some time later, in 1986w1. This microscope has a severe limitation though: it works only on electrically conducting objects, so many interesting materials including biomolecules could not be studied. Binnig and his colleagues continued to search for better solutions, and in 1986, presented the atomic force microscope (AFM), which can be used to image both conducting and non-conducting materials.

The instrument works not unlike a record player, in which a sharp needle scans a vinyl record to reproduce sound (see image, right). The AFM ‘feels’ the atoms, rather than ‘seeing’ them: the structure of a surface is scanned with a very sharp cone (typically of silicon or silicon nitride) at the end of a flexible cantilever that can follow even the smallest details of the surface. When the tip, consisting of a single atom, comes close to the sample surface, it is deflected through forces between the two: these can be mechanical contact forces, van der Waals forces, capillary forces, chemical bonding, electrostatic forces, magnetic forces, Casimir forces, solvation forces or others, depending on the nature of the specimen.
Because this variety of forces can be measured with the AFM, it is very versatile and has led to an explosion in the number of scientists using the instrument – mostly but not only for material sciences and biology. In each case, the force causing the deflection is tiny and proportional to the tip’s distance from the surface.
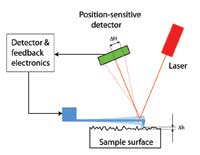
How can these tiny deflections be measured? The inventors used a clever trick: a laser spot is shone onto the top of the cantilever, from where it is reflected onto a position-sensitive light detector. The change in the laser spot’s position on the detector (see diagram on left, ΔH) due to a deflection of the cantilever (see diagram on left, Δh) is proportional to the distance between the detector and cantilever. With large enough distances between them, even tiny deflections can be measured, making it possible to study the structure of surfaces atom by atom.

The applications of the AFM are myriad. Let us take a brief tour of just a few of them. Originally, AFM was developed to observe and analyse surface structures in minute detail – not only is this interesting for research purposes, but it can have direct economic benefits: biofouling is the undesirable accumulation of micro-organisms, plants, algae and / or animals (such as barnacles, Cirripedia) on wet structures. On ship’s hulls, high levels of fouling can increase water resistance and thus substantially increase fuel consumption, but it is also an issue in membrane bioreactors, cooling water cycles of power stations and certain oil pipelines. Scientists use AFM to measure the degree of biofouling and thus compare the anti-biofouling activity of different substances, to identify the ideal material (Finlay et al., 2010).

Similarly, AFM has its uses in agriculture: pineapple plants often suffer from a fungal disease called fusariosis. Scientists compared the surface structure of cells from pineapple cultivars that are resistant to this disease with those of susceptible cultivars, and found that they have different mechanical properties. This can now be used to select and improve resistant cultivars that have the required mechanical properties (de Farias Viégas Aquije et al., 2010).
Is surface structure relevant to human health, too? The answer is yes: AFM studies are often used in dentistry, for example to compare the effectiveness of different methods to remove plaque and stains; to measure the surface roughness of braces and see how this influences the effectiveness with which the teeth are pulled into shape; or to quantify the erosion of tooth enamel caused by acid in soft drinks and test the efficacy of various toothpastes in repairing this damage (Kimyai et al., 2011; Lee et al., 2010; Poggio et al., 2010).
Other medical applications include the development of new biomaterials in regenerative medicine: their surface properties such as wettability, roughness, surface energy, surface charge, chemical functionalities and composition can determine the behaviour of the cells they will come into contact with. Thus AFM can be used, for example, to help design biomaterials that are tolerated by the body and can be used for medical implants such as artificial hips (Al-Ahmad et al., 2010; Kolind et al., 2010; Padial-Molina et al., 2011).
Another major field of application for AFM in medical biology is the misfolding and aggregation of proteins such as α-synuclein, insulin, prions, glucagon and β-amyloid. These phenomena have long been implicated in degenerative diseases such as type II diabetes, Parkinson’s, spongiform encephalopathy (‘mad cow disease’), Huntington’s and Alzheimer’s. Here AFM has already provided important information on the nanoscale structure of the aggregates, and it is hoped that scientists will be able to use AFM to identify why the protein misfolds in the first place, and how it encourages surrounding proteins to adopt the same misfolded structure (Lyubchenko et al., 2010; for an explanation of prion misfolding, see Tatalovic, 2010).

Further biological interactions that have been studied with AFM include how human trophoblasts (cells forming the outer layer of a blastocyst which provide nutrients to the embryo and develop into a large part of the placenta) interact with epithelial cells of the uterus – the basis of successful embryo implantation (Thie et al., 1998).
It was only a small step from using AFM for observations to using it to manipulate atoms, molecules or other nanoscale structures. For example, using the tip as nano-tweezers, precise regions of the cell’s plasma membrane can be examined; individual protein loops can be removed to reveal the protein structure inside the molecule; and single molecules can be stretched into novel conformations to determine their elasticity.
The next big step will be using AFM for nanosurgery: introducing or extracting individual molecules from the cytoplasm of individual cells, to study cellular homeostasis or for subcellular drug delivery (Lamontagne et al., 2008; Müller et al., 2006).
Modified AFM tips can also be used as drills or pens: nano-milling removes material in the form of long curled chips (Gozen & Ozdoganlar, 2010), whereas dip-pen nanolithography is the controlled delivery of molecular or liquid ‘ink’. In chemistry and the life sciences, such technology is used to produce nanoscale sensors or, by the deposition of metallic, semiconductor and metal oxide nanostructures, functional nanocircuits or nanodevices (Basnar & Willner, 2009). This, combined with using the AFM tip to physically push nanometre-sized particles to a desired position, should pave the way for the miniaturisation of electronic circuitry and other structures.
Despite its vast number of applications – these are just a small sample – the possibilities of AFM are not yet exhausted. Future trends involve optimised tips and combinations with other techniques, for example to simultaneously determine surface structure and fluorescence or electrical properties (Müller et al., 2006). Speed is another issue: recently, an AFM has been developed with which biological processes such as chromosome replication and segregation, phagocytosis and protein synthesis can be imaged in real time, up to 1000 times faster than was previously possible (Ando et al., 2008).
Are you now itching to come up with your own applications for AFM? Then you might want to try and follow Philippe Jeanjacquot’s instructionsw2 for building your own instrument at school. It is a time-consuming project, but he and his students managed to create a feasibly low-cost microscope. There is one important catch though: you will need a vibration-free environment to set it up, such as a quiet cellar. If you can find that, your enthusiasm and ingenuity are the only limitations.
This article would be suitable for a wide range of science lessons – not only in physics but also when considering animal physiology or biomedical sciences, for example. Students can research atomic force microscopy and its uses further, as there is plenty of material on the Internet about the advantages and disadvantages of various microscopy techniques. They could also look up the group of scientists who invented the atomic force microscope (having won a Nobel Prize for a previous invention) and find out more about them and their work.
Potential comprehension questions include:
The article could be used with groups of older students or those most able to think creatively, perhaps for an extended writing task in conjunction with the film Honey I Shrunk the Kids (about a scientist working on a top-secret machine that miniaturises objects and – accidentally – people), to get the students to think about looking at single molecules. What would they like to use AFM for? Would they use their images as art or for scientific research? Would they want to use their knowledge to cure diseases or to see how beautiful science can be at this level?
Jennie Hargreaves, UK
AFM instructions (Word document)
Download this article as a PDF