Bringing the beauty of proteins to the classroom: the PDB Art Project
The PDB Art project aims to make science more accessible and inspire young people to explore the beauty of proteins by bringing together art and…

The following was adapted from an ESRF News article.
You shall not pass: discover how the protein coating around an egg cell ‘zips up’ after fertilization to stop more sperm from entering.
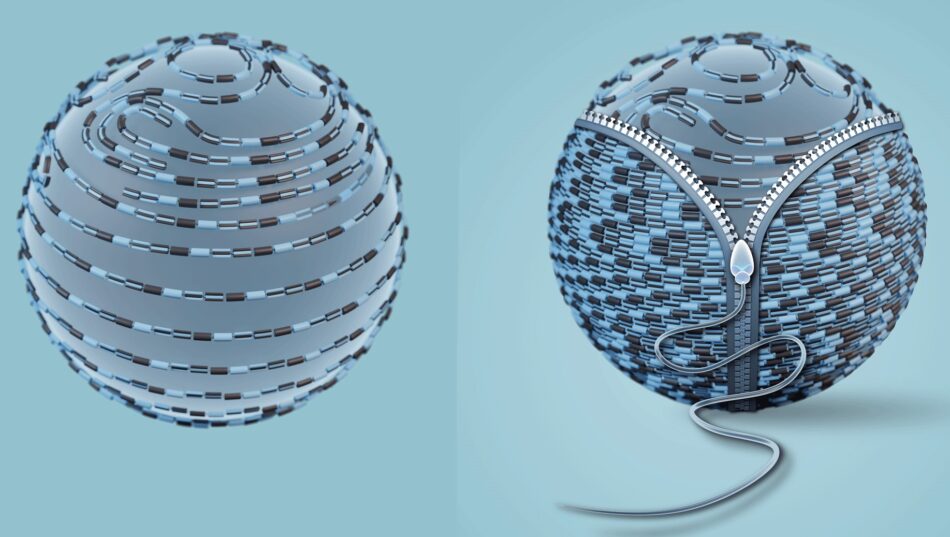
Infertility affects around 17.5% of the adult population worldwide, according to the World Health Organization (WHO). There are many possible causes and either partner can be affected. Some cases of infertility are associated with mutations in the genes that are responsible for egg coat formation. The egg coat, also known as the zona pellucida (ZP), is an extracellular matrix essential for egg growth, fertilization, and protection of the embryo until it implants into the uterus. The ZP consists of several proteins, which polymerize into long filaments that form a ‘mesh’ around the egg.
Mutations in ZP proteins may cause defects in or lack of this mesh. It is currently unclear how many infertile women carry pathogenic mutations in ZP genes because not all of them have their genome sequenced. However, several different types of ZP mutation have been reported to date.
To understand how these mutations affect fertility, we need to understand the processes that take place during fertilization at the molecular level. A team of researchers have discovered how ZP filament rearrangement prevents the fertilization of an egg by more than one sperm (polyspermy), which is generally lethal to the embryo. Scientists already knew that, after a sperm fuses with the egg, the egg releases an enzyme that specifically cleaves a major ZP protein called ZP2. It was also known that the egg coat becomes ‘harder’ after fertilization and additional sperm then can’t penetrate it. However, the connection between these processes and the basis of the block to polyspermy remained unclear. “We knew that these events were associated, but a molecular mechanism linking the different pieces of the puzzle was missing”, explains Prof. Luca Jovine, leader of the study.
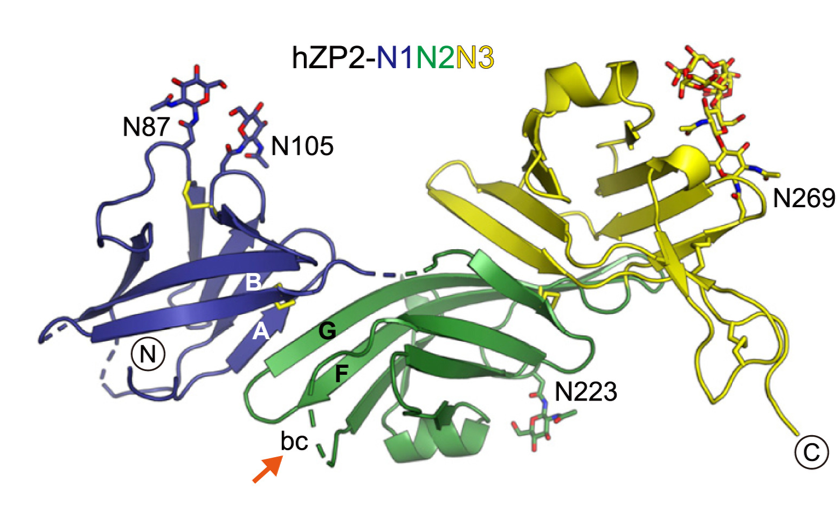
The team aimed to investigate the structure of ZP2 before and after cleavage triggered by egg–sperm fusion, and how ZP2 cleavage changes the overall structure of the ZP mesh. To do so, they examined different ZP2 and egg coat filament structures by using X-ray crystallography at the ESRF, Diamond Light Source, and BESSY II, as well as cryo-electron microscopy at SciLifeLab. According to Daniele de Sanctis, scientist in charge of beamline ID29 at the ESRF, “Seeing the atomic details of such a fundamental process of life is simply amazing.”

Jovine and colleagues discovered that cleavage of the part of ZP2 that protrudes from the egg coat filament (see figure 2) allows it to form new interactions with other ZP2 molecules. These interactions create an extensive network of cross-links that bring the filaments closer to each other. By stiffening the egg coat and tightening its mesh, this prevents additional sperm from penetrating the ZP. It is like a zip that is being done up.
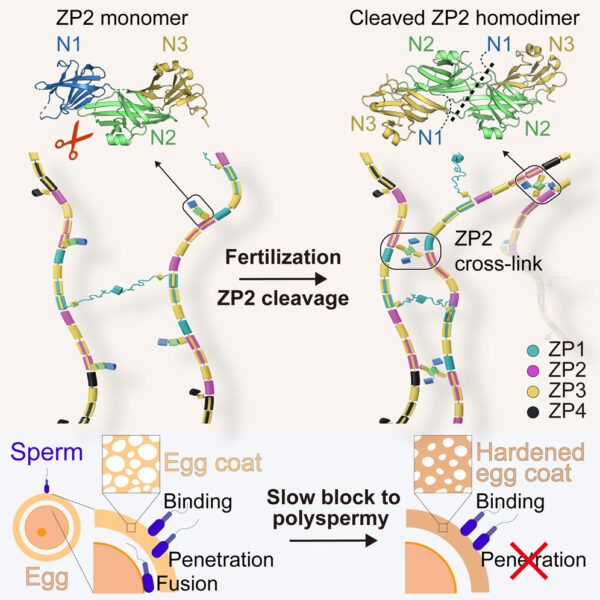
The system does not block polyspermy by preventing sperm binding to the ZP (as previously believed), but rather by hindering sperm penetration of the egg coat. The mechanical system that we discovered is very logical from an evolutionary point of view because it permanently blocks sperm in a way that does not depend on how they attach to the egg coat, which may vary in different species.
Although these findings are at a fundamental level, they have important implications for future reproductive medicine: for the first time we have a molecular view of how the egg coat changes its architecture after fertilization and how this affects its function. This knowledge allows us to interpret a growing number of human ZP gene mutations linked with female infertility. Furthermore, since the egg coat is required for natural fertilization (though not in vitro fertilization), detailed information on its structure could have diagnostic applications and may, in principle, be exploited to develop non-hormonal contraceptives.
The ESRF structural biology beamlines have played a very important role in this study. In 2017, they allowed Jovine and de Sanctis to provide a first example of how egg coat and sperm recognize each other at the beginning of fertilization and, in 2010, to solve the 3D structure of ZP3, another major egg coat subunit that is also implicated in sperm binding. New experiments that will be made possible by the new Extremely Brilliant Source at the ESRF are already being planned.

The ESRF is the world’s brightest synchrotron light source. Every year, 9000 scientists from 20 partner countries and from around the world travel to Grenoble to use its extremely brilliant X-rays for leading-edge research. This fundamental and applied research contributes to addressing the complex global challenges that our society faces, such as health, energy, and the environment. It also contributes to the development of new technologies for industry and to preserving humanity’s cultural heritage, lighting the way to a sustainable and peaceful future.
The strength of the ESRF is its capacity to innovate, pushing the limits and seeking ever-higher performance for the benefit of the global scientific community. In 2020, the ESRF launched a brand-new generation of high-energy synchrotron, the ESRF’s Extremely Brilliant Source (EBS). With the support of the ESRF’s 20 partner countries, EBS provides scientists with new opportunities to unveil the structure of materials and the mechanisms of life, down to atomic resolution.
[1] Nishio S et al (2024) ZP2 cleavage blocks polyspermy by modulating the architecture of the egg coat. Cell 187: 1440–1459. doi: 10.1016/j.cell.2024.02.013

The PDB Art project aims to make science more accessible and inspire young people to explore the beauty of proteins by bringing together art and…

The asteroid that killed the dinosaurs struck Earth during springtime. Scientists have determined this by analyzing the remains of fish that died…
Lactase tablets for managing lactose intolerance can be used in the classroom to explore the biochemistry of sugars and the properties of enzymes.