
Inclusive lesson plans using the NinU grid
The idea of ‘science for all’ can only be accomplished when we recognize the need to embrace diversity, reduce barriers, and enable participation…

Discover simple adaptations to apparatus and experiments that make practical chemistry more accessible to students with vision impairment.
Chemistry can be challenging for pupils with vision impairment (VI) because it involves visual laboratory observations, quantitative dispensing of chemicals, and complex diagrams. Pupils with VI are often excluded from active participation in laboratory experiments, with sighted partners or assistants carrying out the experiment instead. Some specialist equipment is available, such as talking thermometers or timers. However, these can contribute to a feeling of self-consciousness or otherness.
Inclusive education aims to allow all students to learn in the same environment.[1] To allow diverse groups to learn side by side, it is important to design experiments and apparatus that adhere to universal design. This means creating products and activities that are accessible to all without the need for adaptation. In the specific case of pupils with VI, universal design allows them to participate alongside their sighted peers in scientific experiments. Multisensory experiments may also enhance understanding for sighted students.
There are simple ways to adapt existing experiments to make them accessible for students with VI and allow them to work independently. Compared with observing an assistant perform the experiments, this allows VI students to feel more engaged and enthusiastic.
Weighing reagents is a significant challenge for pupils with VI. Talking balances are available but these are designed for weighing large masses in the kitchen. Since they are also costly, they would have to be used only by the pupil with VI, which would contribute to a feeling of otherness. An alternative option is for teachers/technicians to determine the equivalent volume of a material that corresponds to a desired mass. For example, tablespoons of activated carbon, scraped level with the flat back of a knife, provide a consistent mass (figure 1). Kitchen measuring spoons are readily available and can be provided to the whole class at low cost. If all students use the same spoons, then no student feels different. It should be noted that larger grain materials are not as consistent in mass when measured in very small spoons.
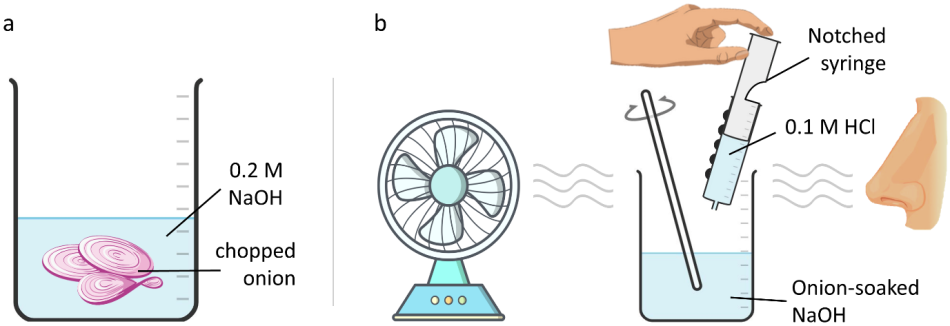
Adding dye to solutions to increase contrast, or using large print or tactile labels on measuring apparatus, can make it easier for pupils with VI to take measurements. However, these adaptations still rely on partial sight. We have developed a simple and effective alternative to this. Notched syringes (figure 2) can be made to measure a specific volume. It would be possible to cut multiple notches to allow one syringe to be used for multiple volumes, but this could cause confusion.[2]
The procedure for making a notched syringe is as follows:
The procedure for using the syringes is as follows:
Pupils with VI said that these syringes would allow them to “perform practical work without assistance” and to be “confident in science.”
In addition to adapting equipment for measurement, it is possible to design experiments that use senses other than eyesight.
There are a range of existing experiments with an olfactory component that lend themselves naturally to inclusivity and universal design, such as esterification. The kinetics of ester formation by Fischer synthesis can be explored by varying the alcohol and carboxylic acid structures and monitoring the rate at which the ester can be smelt.[3]
ChemBAM-VI is a project that was set up to provide experiments and activities that are inclusive to students with VI. One experiment we created as part of the ChemBAM-VI project compares activated carbon and BBQ charcoal for removing fragrances from water.[4] This is a way to introduce concepts such as water treatment, porosity, adsorption, and intermolecular interactions. The same experiment can be done with coloured dyes, but the olfactory version is accessible to pupils who are sighted or who have VI.
Additionally, we developed an olfactory titration by using onions as the indicator for an NaOH–HCl titration.[5] The experimental setup can be seen in figure 3.
Titration ColorCam is an app that can convert the colour change in titration experiments into beep sounds and vibrational pulses.[7] It is effective at detecting colour changes of specific indicators and informs users before and upon reaching the end point.
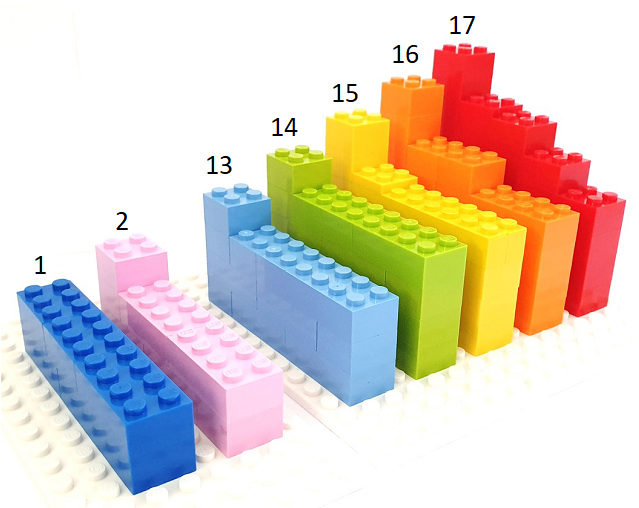
Compared with vision and hearing, touch has been found to be superior for processing material characteristics and the detailed shapes of objects. LEGO bricks can be used as a tactile teaching resource to teach concepts in chemistry such as periodic trends, molecular orbital diagrams, and electron configuration, as shown in figure 4. This simple yet effective initiative shows the potential for using low-cost household items as teaching resources.
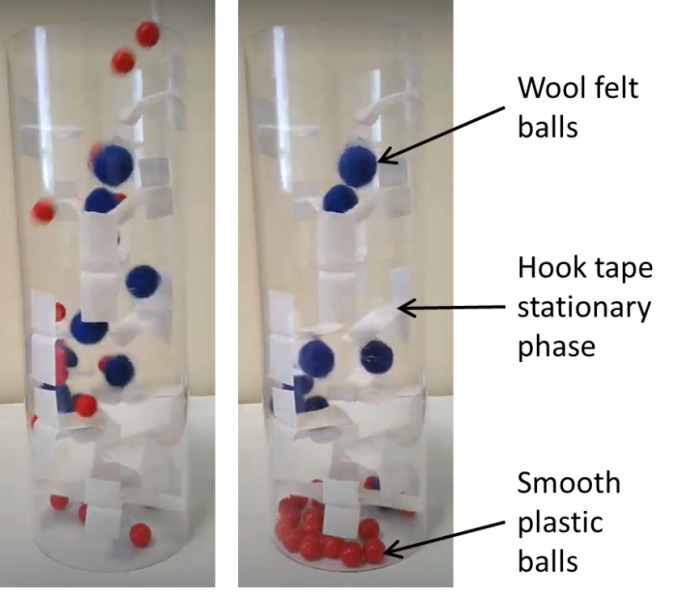
As part of the ChemBAM-VI project, we have designed a tactile chromatography column.[8] Plastic balls and wool felt balls illustrate the mixed components and the strips of hook tape lining the plastic tube represent the stationary phase (figure 5). Wool felt balls stick to the tape, while smooth plastic balls fall through. The balls that have fallen through can be felt and the column is large enough that it can also be explored by hand. This allows students to determine which material has interacted with the hook tape. This can be used to explain the concept of column chromatography and mixture separation to sighted students and those with VI.
Teachers may assume that VI students cannot take part in most chemistry experiments, meaning these students often rely on assistant support. Integrating students with VI into practical classes by running experiments that use senses other than sight can add great value to their science education. Simple adaptations that allow pupils with VI to participate more actively in laboratory work can be worthwhile and meaningful. Not only do students with VI benefit from feeling included in the laboratory, but the experiments enhance understanding and memorization for sighted students by offering them additional approaches to understanding the content.

ChemBAM is an outreach project run by chemists at the University of Birmingham. The project is focused on bringing chemistry to life by linking the curriculum to real-world chemistry. ChemBAM-VI is a special project that aims to make chemistry accessible for pupils with vision impairment.
[1] The UNICEF page on inclusive education: https://www.unicef.org/education/inclusive-education
[2] Measuring volume instructions for students with VI on the ChemBAM website: https://chembam.com/vi/general-tips/measuring-volume/
[3] The Fischer esterification reaction adapted for students with VI by monitoring the ester smell: https://chembam.com/vi/kinetics-of-ester-formation-via-fischer-esterification/
[4] Instruction on removing an organic fragrance molecule from water to demonstrate the ability of activated carbon to remove pollutants: https://chembam.com/vi/carbons-for-water-treatment/
[5] A titration reaction for students with VI uses onions as multisensory indicators: https://chembam.com/vi/olfactory-titration-experiment/
[6] Chataway-Green R, Schnepp Z (2022) Inclusion: An accessible olfactory titration experiment for the visually impaired. SSR in Practice 386: 32–33.
[7] Bandyopadhyay S, Rathod BB (2017) The Sound and Feel of Titrations: A Smartphone Aid for Color-Blind and Visually Impaired Students. J. Chem. Educ. 94: 946–949. doi: 10.1021/acs.jchemed.7b00027
[8] A chromatography demonstration for students with VI: https://chembam.com/vi/tactile-models/tactile-chromatography/
Equal access to education must be a matter of course for all learners – regardless to individual abilities. Still, safety must be guaranteed at all times for work in the chemical laboratory. This article presents innovative and easy-to-implement ways to make self-directed chemistry experimentation accessible to more students, regardless of their abilities.
Katja Weirauch, lecturer for Chemistry Education, University of Würzburg, Germany

The idea of ‘science for all’ can only be accomplished when we recognize the need to embrace diversity, reduce barriers, and enable participation…

Thinking outside the box: explore the nature of science by building LEGO mystery boxes and challenging your students to solve the…

Play the part: students take on the roles of different components of a synapse to act out synaptic transmission and learn about neurobiology.