Supporting materials
Download
Download this article as a PDF

When talking of finite resources, the chemical elements themselves are often overlooked. Learn more about elements in danger.
We hear so much about the very real damage that increasing levels of certain chemicals can have on our planet, for example, high levels of CO2 in the atmosphere or plastic in the oceans. However, we rarely hear about how the decreasing availability of some chemicals could alter our lives. It might surprise you to know that many elements critical to our technological society are in short supply.
Elements are the simplest chemicals and generally cannot be created synthetically (at least not in significant quantities). They are a finite resource and once we have exhausted our planet’s supply, there is currently no realistic way of obtaining more. Recent studies on elemental sources in the Earth’s crust have found that up to 35 elements are ‘in danger’.
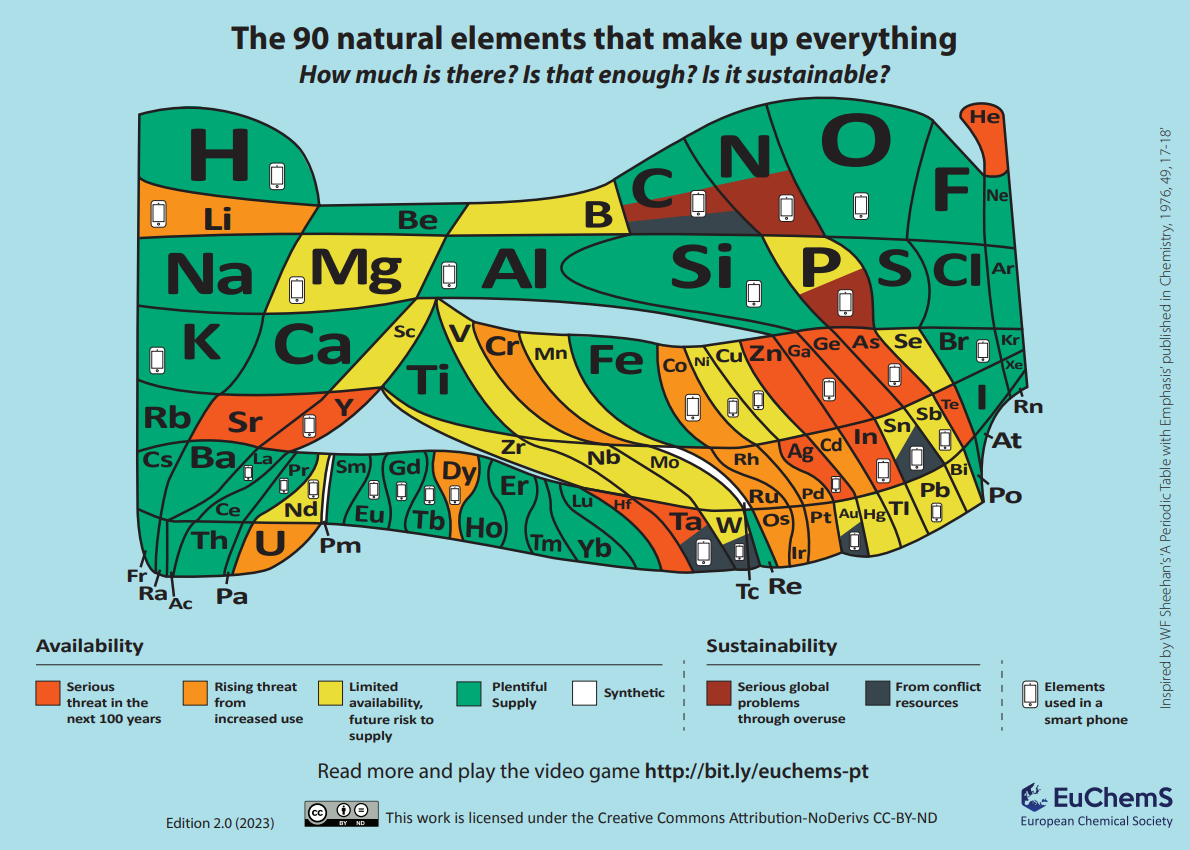
In this article, we explain why some elements are considered to be in danger, consider some of the technology that relies on the properties of these elements, and aim to give some hope for the future.
Some important elements that we need for various applications are becoming scarce for several reasons.
| Reason for criticality | Example elements | Uses |
|---|---|---|
| Low abundance in the Earth’s crust | Helium | MRI scanners, cryogenics |
| Challenging/expensive to extract | Rare earth elements | EVERY electronic device, renewable energy applications |
| Dangerous to mine | Lanthanum | Hybrid cars, refined petroleum |
| Environmentally damaging to mine | All mined ores e.g. those of yttrium and scandium | MANY purposes |
| Only obtained as a byproduct | Gallium | Semiconductors used in microchips |
| Vulnerable to loss of supply and conflict mining | Cobalt and rare earth elements | Lithium ion batteries |
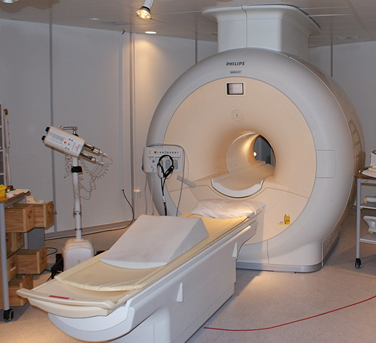
An example of an element of low abundance is helium. Helium is the second most abundant element in the visible universe, having been being created in stars through nuclear fusion for billions of years. However, it is an example of an element in low abundance on Earth due to its low density and inert nature, with supplies starting to run low. Liquid helium has many important uses, including within MRI scanners.
The rest of the elements in danger are metals obtained through the mining and processing of mineral ores. An example is rare earth elements (REEs), which are transition metals mostly located in the lanthanide series. One or more REEs are found in every electronic device, and most people in developed countries now have multiple electronic devices, with 10 million new phones bought every month in the EU alone.[1] REEs aren’t found in high concentrations in minerals, so they are challenging and expensive to obtain in large quantities.
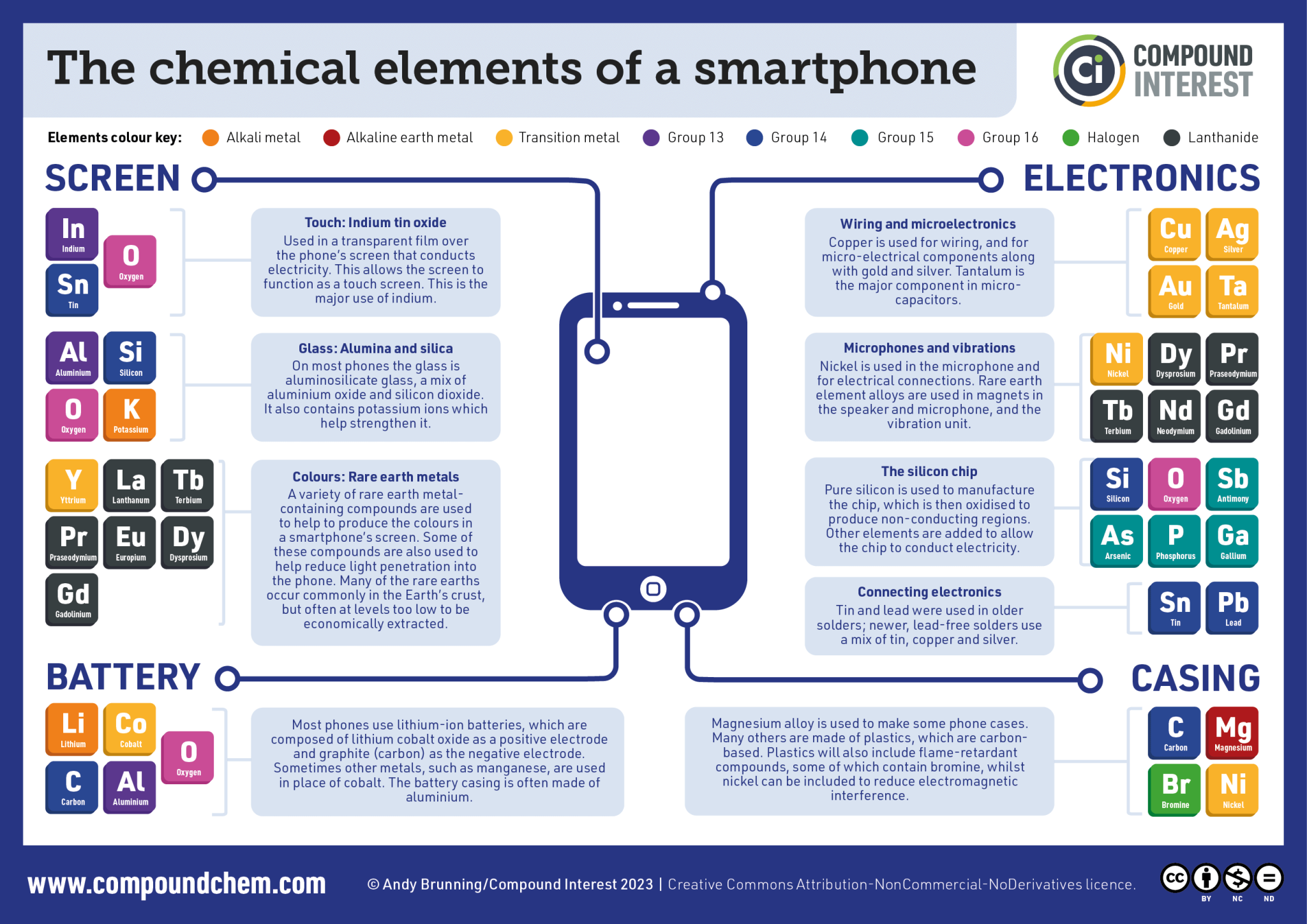
Even lowering carbon emissions has a darker side. Technologies for renewable energy often use REEs as well as other metals. Neodymium, praseodymium, and dysprosium are all used within the electricity generators in wind-turbines. Furthermore, indium and gallium (which are not REEs but are also challenging to extract) are used as dopants to modify the properties of semiconductors, which are used widely in the manufacture of solar panels.
As global demand for alternatives to fossil fuels rises, the demand for these metals is also predicted to increase.
Photovoltaic solar energy [Box 1 in the Supporting Information] requires 13 t of indium and 4 t of gallium per gigawatt (GW). For wind energy, 200 t neodymium and 13 t dysprosium are currently required per GW.[2] The demand for these elements is expected to increase by as much as 700% and 2600% for neodymium and dysprosium, respectively, in the next 25 years.[3]
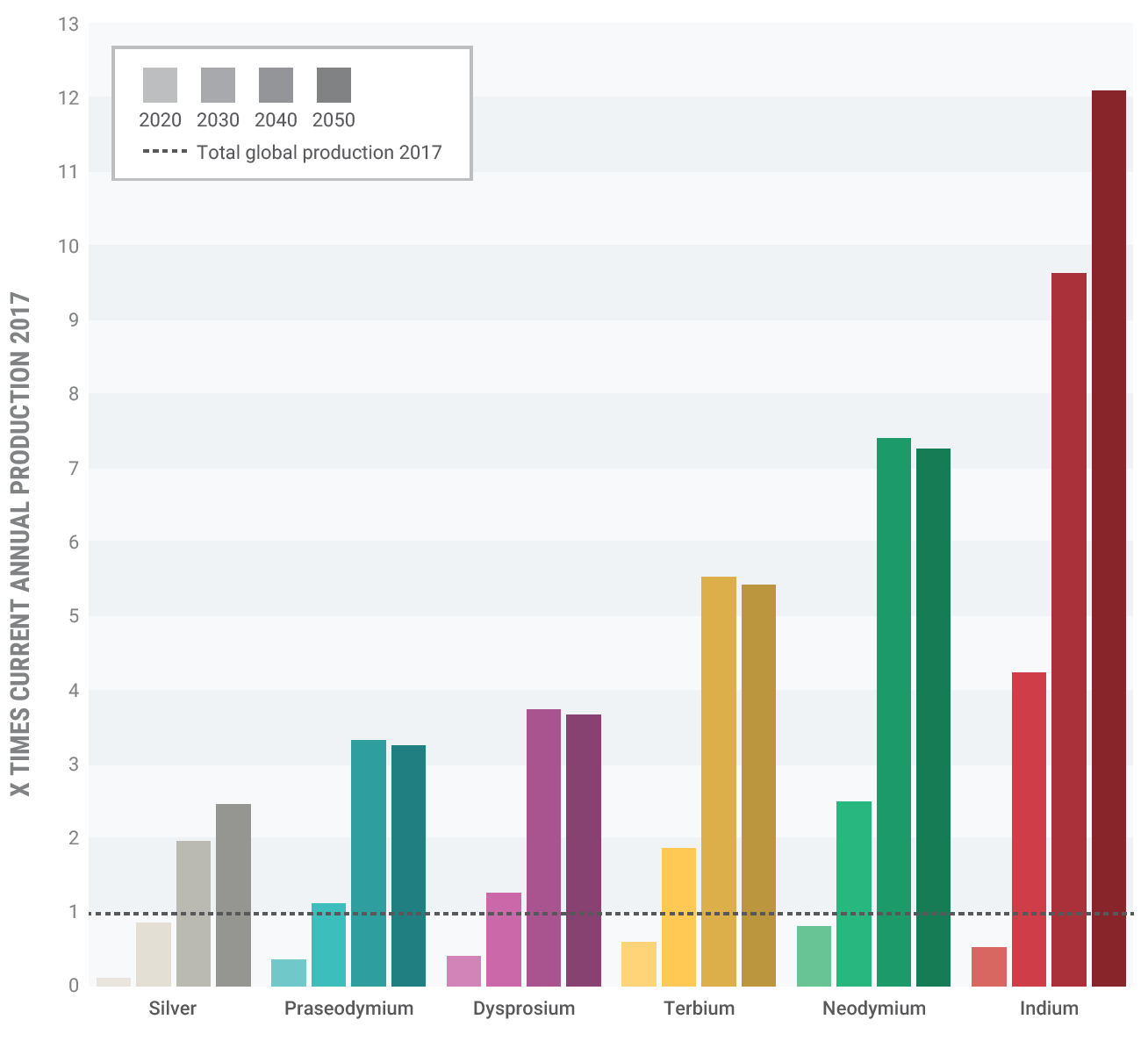
The mineral monazite contains lanthanum but also the radioactive elements thorium and uranium. Lanthanum is a key component in current hybrid car batteries (averaging 15 kg per car [4]) [Box 2] and is used in catalysts for petroleum refining. Since the ore is radioactive, mining monazite is inherently dangerous and costly, and the storage, transportation, and disposal of waste materials are all more expensive.

Mining minerals is often environmentally damaging since it frequently involves removing vegetation and trees, eroding the rocks, and polluting local water supplies. Historically, this was particularly problematic for REEs since unregulated REE mining was practised to keep the prices low, and contaminated liquids used to extract the REEs were stored in open-topped pools, from which they can leach into the water supply when landslides occur. This previously occurred with all REEs, however stricter regulation in more recent years has reduced this problem.
There are no gallium mines because the concentration of gallium in ores is very low. Gallium is obtained as a byproduct of aluminium [Box 3] and zinc smelting and is used to manufacture semiconductors, which are used in many electronic devices, with 10 times more gallium used in current smart phones relative to older models.[5] If you want more gallium, you need to smelt more aluminium or zinc, which releases CO2 (760 megatonnes/year for aluminium [6, 7]) and would also cause the prices of aluminium and zinc to drop, causing economic challenges.
Many REEs are produced predominantly in particular parts of the world. This can lead to supply issues if there is political instability, or the producing country decides to limit supply. Shortages result in price hikes, and these rapid changes cause chaos in the technology and finance worlds.
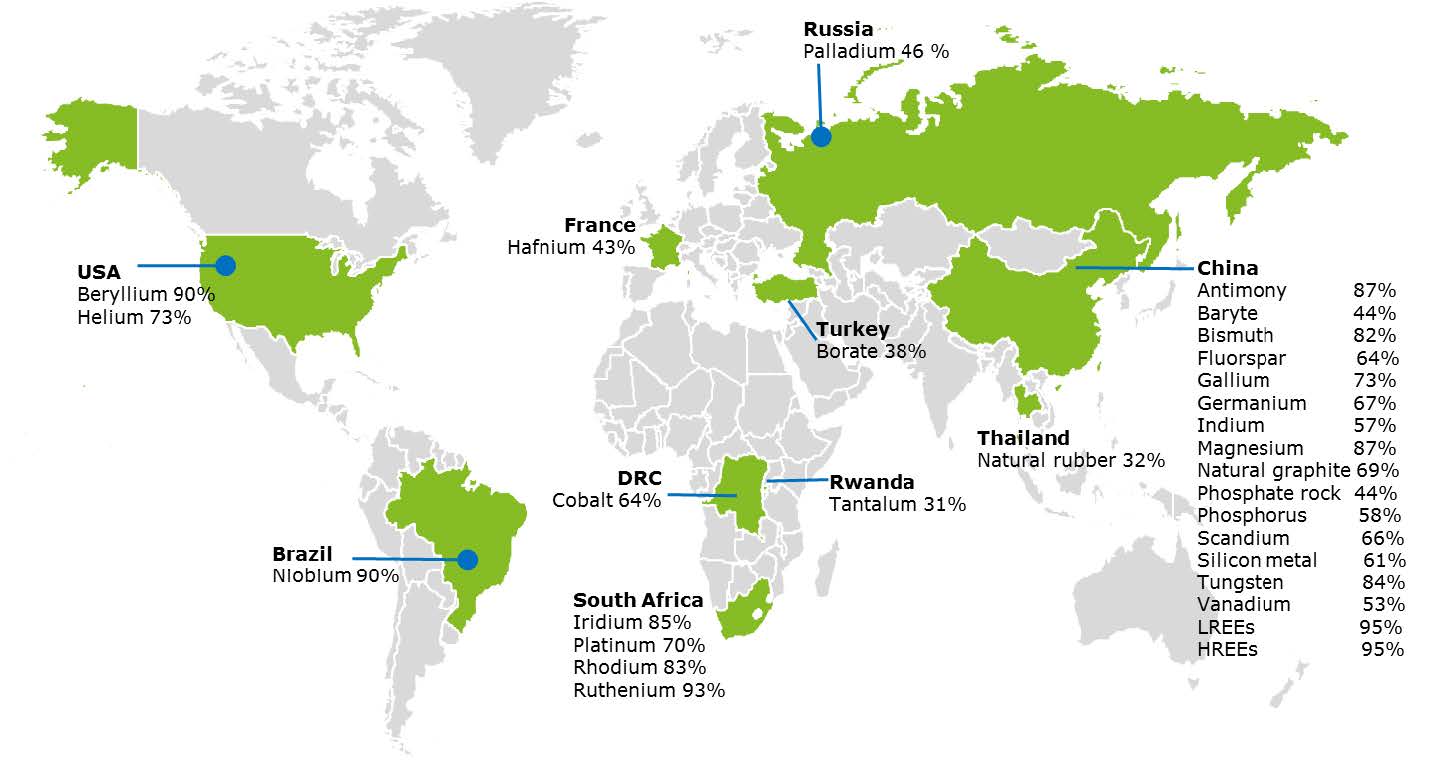
There is also the issue of conflict resources: materials that often rely on child or slave labour, or dangerous unregulated mining conditions and techniques. The profits might also be used to finance brutal conflicts. Cobalt, an important part of most lithium ion batteries [Box 4], along with tantalum and tungsten, are known to be conflict resources. Governments are starting to impose more stringent laws upon technology companies, with ‘material passports’ to reduce conflict mining.
One of the main purposes of science is to find solutions to current challenges, and it is doing just that! We can reduce, re-use, recycle, substitute, improve manufacturing, or find new sources.
One of the first things we can do is reduce unnecessary consumption. We are conditioned by advertisers to want the latest gadget, but your current model can easily last longer than a few years. If you do give in to your inner ‘tech-nerd’, don’t throw the old one away or forget it in a drawer.
Recycling:

2. Reduces the need for environmentally damaging mining
3. Can happen anywhere
4. Can generate local jobs
Some elements are widely recycled, such as tungsten, which is used in the manufacture of mining and construction tools. However, this is not the case for most of the elements in criticality, 77% of which are only recycled 10% of the time.[8] Often this is because the element is used in the product in trace quantities and is not easy to recover. These small quantities add up, with an estimate in 2018 of approximately 1.2 billion tonnes of electronic waste (containing precious REEs and metals) not being recycled and instead entering landfill.[9]

Efforts are being made to increase the recycling of these critical elements, however, this is an expensive process. A shift in the world’s attitudes towards recycling these materials is urgently required.

Current research is focusing on how to substitute with elements not on the critical list, such as the move to use lithium-iron-phosphate batteries rather than nickel-cobalt-aluminium [Box 2] for electric cars. However, many critical elements are not easily replaced due to their unique properties.
New sources are needed and there are several under development:
• Processing coal[10] before use. Small quantities of REEs are found in coal and can be extracted through ion-exchange techniques.
• Urban landfill mining.[11] This is not currently profitable because the concentrations of each metal in landfill are too low, however, it should eventually become more viable.
• Phytomining.[12] Some plants are known to be hyperaccumulators, concentrating metals obtained from the soil within their cells. When planted in soil that contains metal ore or waste, the plants absorb the metals, which can then be extracted.
The cheapest option is still mining though, and companies are conducting surveys on the ocean floor to see if it could be tapped for more resources. However, deep-sea mining[13] would clearly create environmentally damaging effects.

Possibly. Asteroids are lumps of mineral-rich rock that could be mined. Asteroid mining[14] may sound like wildly far-fetched science fiction, however, there are companies seeking to do just this. NASA even sought the possibility in the 2018 USA federal budget. This would, however, be very expensive.
All in all, we are not yet at a stage for worldwide panic over the loss or dwindling supply of specific elements, however, we could be a lot closer than we would care to admit. A good place to start is to try and reduce unnecessary consumption, look after the planet, and keep learning!
[1] A news piece on the elements in danger by David Cole-Hamilton for the Royal Society of Chemistry: https://www.rsc.org/news-events/opinions/2019/jan/elements-in-danger
[2] Månberger A, Stenqvist B (2018) Global metal flows in the renewable energy transition: Exploring the effects of substitutes, technological mix and development. Energy Policy 119:226–241. doi 10.1016/j.enpol.2018.04.056
[3] Alonso E (2012) Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environmental science and technology 46:3406–3414. doi: 1021/es203518d
[4] An article on rare Earth metals and hybrid cars by the Kidela Capital Group: https://www.mining.com/rare-earth-metals-and-hybrid-cars/
[5] S. Geological Survey, Mineral Commodity Summaries (2018), Gallium, USGS.
[6] Article on the aluminium industry’s race to zero carbon: https://aluminiuminsider.com/leaders-emerge-in-the-aluminium-industrys-race-to-zero-carbon/
[7] Van Genderen E et al. (2016) A global life cycle assessment for primary zinc production. The International Journal of Life Cycle Assessment 21:1580–1593. doi: 1007/s11367-016-1131-8
[8] (2017) Study on the review of the list of critical raw materials. EU commission, Brussels.
[9] A Forbes article on agromining: https://www.forbes.com/sites/aminmirkouei/2021/05/04/a-sustainable-way-to-mine-rare-earth-elements-from-old-tech-devices-agromining-explained/
[10] An article on Science alert about extracting rare earth elements from coal waste: https://www.sciencealert.com/scientists-figure-out-a-cheaper-more-efficient-way-to-extract-rare-earth-elements-from-coal
[11] Tercero Espinoza L et al. (2020) The promise and limits of Urban Mining. Fraunhofer ISI, Karlsruhe.
[12] An article on the The University of Queensland’s website about phytomining https://smi.uq.edu.au/leaders-energy-transition-sustainable-source-critical-metals-phytomining
[13] A News article on Nature about seabed mining: https://www.nature.com/articles/d41586-019-02242-y
[14] Asteroid mining in an article on Interesting Engineering: https://interestingengineering.com/asteroid-mining-to-shape-the-future-of-our-wealth
Download this article as a PDF