An artistic introduction to anthocyanin inks Teach article
Making pH-sensitive inks from fruits and vegetables is a creative variation of the cabbage-indicator experiment.
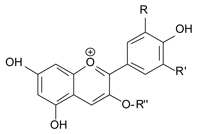
anthocyanin, in which R and
R’ are H, OH, or OCH3 and R”
is a sugar unit
Image courtesy of Gustavo
Giraldi Shimamoto and Adriana
Vitorino Rossi
Long before synthetic dyes and pigments were available, natural products were used to create simple but efficient inks (such as that described by Farusi, 2012) – which we can still see in the paintings of prehistoric and extinct civilisations. These natural colours could be mineral in origin or from plant extracts; in this activity we focus on a particular group of plants that contain anthocyanins (ACY, figure 1).
Natural colours are often less stable than many synthetic dyes, changing colour or disintegrating with temperature, light, pH variation and oxidising agents. This can lead to interesting lessons, and in our work with primary school students we have used anthocyanins as colourants for pH-reactive ink production.
The origin of colour
Anthocyanins are organic compounds that produce the orange, red, purple, blue and nearly black colours in the flowers, fruit, leaves and roots of several fruit and vegetable species. Anthocyanin molecules contain ring structures that absorb light, and so act like a sunscreen, protecting the cells of the plant. The wavelengths of light that are not absorbed by the anthocyanin are instead reflected, and so the flower or fruit is seen as coloured. These colours also attract pollinators and seed dispersers and so are also important for the plant’s reproduction.
Anthocyanin solutions change colour with the pH of the medium, acting as a natural pH indicator (figure 2). Generally, anthocyanins exhibit a red colour in acid solutions and a blue colour in alkaline media; however, other colours can be observed, depending on the pH and the particular anthocyanin and its source. This is because adding and removing hydrogen ions (H+) from the pigment’s molecular structure changes its electronic properties and so alters the wavelengths of light that the pigment absorbs. This property allows us to use plant extracts to determine the pH of household materials and to indicate the endpoint of acid/base titrations, as in the famous cabbage-indicator experiment (Terci & Rossi, 2002; Rossi & Shimamoto, 2010). The colour variability of anthocyanins can also be explored by producing ink for brushes, stamp painting or screen printing.

Image courtesy of Gustavo Giraldi Shimamoto and Adriana Vitorino Rossi
What is an ink?
Inks are mixtures of dyes or pigments and a binder compound, which helps the ink stick to the surface to be painted. This trivial formulation has been used for thousands of years, but advances in chemistry and new technological alternatives have made inks much more sophisticated, with infinite colour options and diverse properties, such as the ability to adhere to glossy paper or egg shells.
An essential component of an ink is the substance that imparts the colour: the pigment or dye. A pigment is a finely divided solid that is insoluble in the dispersion medium of the ink and provides – in addition to colour – opacity and strength, among other effects, whereas dyes are generally compounds that are soluble in the ink medium. In our ink, the anthocyanin extract is a pigment.
Anthocyanin ink for painting, stamps, or screen printing
Materials

paintings with vinegar solution
Image courtesy of Gustavo
Giraldi Shimamoto and Adriana
Vitorino Rossi
- Fruits or vegetables containing anthocyanins, such as blackberries, grapes, strawberries, blueberries, raspberries, or red cabbage.
- 94% ethanol (volume : volume)
- Filter paper (a coffee filter can also be used)
- Petri dishes
- Water
- Bond paper (a high-quality durable writing paper with a density greater than 50 gm-2)
- Stamps, paint roller or brushes
- Vinegar
- Multi-purpose cleaning products with alkaline properties
Procedure
- Crush the fruit or vegetable and mix in ethanol at an approximate ratio of 1 : 3 (weight : volume) for 30 minutes.
- Filter using filter paper and pour the filtrate into Petri dishes.
- Dry the anthocyanin extract by leaving the extract in opened Petri dishes protected from light until the solvent evaporates (2 days). For more rapid evaporation, the dishes can be subjected to cold airflow or stored in a chemical hood or under a range hood.
- After it is dry, mix the dried anthocyanin extract (which has a pasty appearance) with water in a ratio of approximately 1 : 10 (weight : volume).
You can then use the prepared ink with brushes or stamps, or for screen printing. As this ink is a pH indicator, its colour can be changed by the addition of acid or alkaline solutions. A brush could be used to apply an acid solution (vinegar) and an alkaline solution (diluted multi-purpose cleaner) on paper dyed with the ink, or paintings could be sprinkled with acid and alkaline solutions to change their colours. Another option is to write or draw with acid and alkaline solutions, which are colourless, and reveal the message by spraying with anthocyanin ink, simulating an invisible ink. Approximately 1 minute after applying the anthocyanin ink to bond paper, the paintings will be partially dried.
Safety note
Check your local regulations when using acid or alkaline solutions, as safety glasses may be required.
About what happens
different pH solutions.
(a) Initial colour print,
(b) after acid treatment, and
(c) after alkali treatment
Image courtesy of Gustavo
Giraldi Shimamoto and Adriana
Vitorino Rossi
Anthocyanin extracts are natural pH indicators in solution as well as on paper. The change in colour can be explained by the interaction between the anthocyanin-impregnated water molecules on the paper, and the acetic acid from vinegar or the alkali from the multi-purpose cleaner.
The ink’s colour change is linked to a chemical equilibrium (shown in simplified form in figure 2). Anthocyanins are organic compounds that have several substituents on which H+ ions are added or removed at various pH values to produce different coloured solutions. In figure 2, there are three main colours because three media were used with the anthocyanin paints: acid, neutral and alkaline. By using solutions of different pHs, the different dyes can be compared.
The anthocyanin ink can be used by younger children (under 11) for drawing and painting activities. Older children (11+) or supervising adults can use vinegar (acid) and diluted cleaner (alkaline) to change the designs and engage in interactions with the group. You can easily modify the preparation and application of this ink, which does not require toxic reagents and does not generate residues that need to be treated before disposal.
The main purpose of this recreational activity is to arouse curiosity, but because you can use any fruit or vegetable that contains anthocyanins to produce these pH-sensitive inks, an interdisciplinary approach that incorporates environmental education and local values is possible. While making the inks, you can discuss nature, the environment, biodiversity and their rational exploitation to obtain useful products.
Acknowledgement
The authors acknowledge the São Paulo Research Foundation (FAPESP) for financial support.
References
- Farusi G (2012) Indigo: recreating Pharaoh’s dye. Science in School 24: 40–46.
- Rossi AV, Shimamoto GG (2010) Antocianinas e gelo seco para visualizar equilíbrios ácido/base numa abordagem contextualizada. Educació Química EduQ 7: 31–36 [in Portuguese].
- Terci DBL, Rossi AV (2002) Indicadores naturais de pH: usar papel ou solução? Química Nova 25: 684–688 [in Portuguese].
Web References
- w1 – More information about the Group for Research in Analytical Chemistry and Education at the University of Campinas (UNICAMP) in São Paolo, Brazil.
Resources
- Beautiful Chemistry, a website based in China, has videos and pictures of plants changing colour with pH.
- Anthocyanins can also be used to make dye-sensitised solar cells, as described in this teaching activity:
- Shallcross D, Harrison T, Henshaw S, Sellou L (2009) Looking to the heavens: climate change experiments. Science in School 12: 34–39.
- If you enjoyed reading this article, you may find the following book chapter interesting:
- Salamão AA, et al (2010) Jogo pedagógico que explora a propriedade indicadora de pH de extratos de antocianinas de espécies brasileiras. In de Rezende CM, Braibante HTS (eds) A Química Perto de Você – Experimentos de baixo custo para a sala de aula do Ensino Fundamental e Médio pp35–43. Cidade Universitária, São Paulo, Brazil: Sociedade Brasileira de Química. ISBN: 9788564099005 [in Portuguese]. This e-book is freely available to download.
Review
This is a novel way of teaching old science, either in class or through a science club.
The introduction to the article could be used in a comprehension exercise and the article could be used to stimulate discussion around acids, alkalis and indicators; chemical extraction; plant science; and science and art.
Tim Harrison, Bristol ChemLabS, University of Bristol, UK





