Supporting materials
Download
Download this article as a PDF

Do you enjoy the drama of science? The colour, the smells, the intricacies? Why not follow science teacher Bernhard Sturm’s suggestions: let your students bring yet more drama into the classroom by (re-)enacting science, to help them visualise and remember the lesson.

Drama can combine elements of art, music and sport, and develop students’ creativity and fitness as well as their emotional and aesthetic awareness. As team activities, they also promote communication and co-operation among young learners. So why not use drama in science teaching?
This article offers a selection of drama-based activities to act or re-enact science in the chemistry and physics classroom.
This is a method to visualise the exchange of oxygen between different metal atoms within the redox series (after Lavoisier). Each student wears a top in one of three colours, representing atoms of oxygen and two different metals (there should be equal numbers of each colour). In groups of about 8-10, the students enact simple redox reactions with 1:1 stoichiometry, such as CuO + Fe → Cu + FeO, and afterwards present them to the entire class. Students often find creative ways to represent both the activation energy and the release of energy in these reactions.
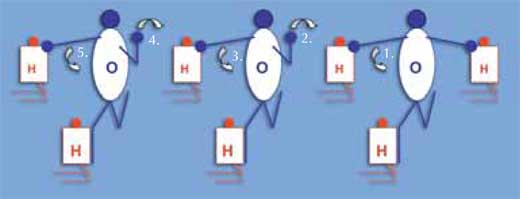
The following method can be used to demonstrate how an electric current passes rapidly through water, although the protons do not travel: instead, a positive charge is transmitted. The students should wear tops in one of two colours, representing hydrogen and oxygen atoms (ratio 2:1). Arrange a line of water molecules: each molecule consists of two (kneeling) hydrogen atoms and one (standing) oxygen atom. The oxygen atom touches one hydrogen atom with his or her right hand and the other hydrogen atom with his or her right foot (see diagram on the right). The hydrogen atom’s arms and legs have no function. One extra hydrogen atom is attached to the oxygen atom (touched by his or her left hand) at one end of the line (H3O+). The arms and legs of the oxygen students represent electron pairs. The surplus binding electron pair is passed from one end of the line of water molecules to the other, as the oxygen atom in the H3O+ molecule lets go of the hydrogen atom with his or her right hand; instead, the oxygen atom of the neighbouring molecule touches this hydrogen atom with his or her left hand, and so on down the line.
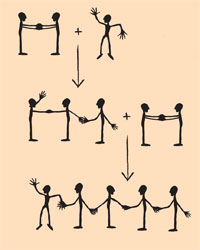
The following method is useful for understanding the mechanism of polymerisation and differentiating between the synthesis of linear and cross-linked polymers. The polymerisation of ethene is illustrated, integrating all students one by one. Each body represents a carbon atom, each foot a hydrogen atom and each arm a binding electron. A single student (representing a methyl radical) waves his or her arm (an unpaired electron). Two students holding both hands (a double bond) represent an ethene molecule. The radical binds to the ethene molecule: one of the components of the ethene molecule has to break one handclasp (binding electron) and bind to the free electron of the radical (new handclasp). The resulting molecule with three carbon atoms is again a radical. It binds another ethene molecule, and so on (see diagram on the left).
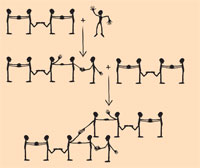
This model can be extended to represent the cross-linking of buta-1,3-diene: the students representing the C2 and C3 atoms of the molecule touch feet to symbolise the single bond (see diagram on the left). The arrival of a methyl radical causes one of the double bonds (double handclasps) in the buta-1,3-diene molecule to break, and a new single bond (handclasp) is formed between the methyl radical and, for example, the C4 atom. The newly formed molecule is also a radical, with a free electron at C3. The C1 atom of a second buta-1,3-diene molecule can thus bind, breaking its double bond (double handclasp) and forming a cross-link (handclasp) with C3 of the original molecule. The resultant nine-carbon molecule is also a radical, with a free binding electron at C2 of the second buta-1,3-diene molecule.
The spacing and velocity of water molecules differs between solid, liquid and gaseous states. In this exercise, students act as water molecules. In my experience, it is best done outside, where there is more space, and it helps to separate the class into male and female teenagers – one group acts, the other watches. The teacher tells the students how to move around: beginning with winter (0 °C), the students should stand still and in a grid formation. As the year goes on, it becomes spring and summer, and the molecules move faster (up to 40 °C), but still stay in contact. Finally, the molecules end up in a kettle, heat up and evaporate (100 °C).
In each phase, the teacher takes a snapshot of the state by shouting “Stop!”. Actors (e.g. the girls) and spectators (e.g. the boys) describe what was happening before and what they can see around themselves now.
The groups of actors and spectators then swap roles, and the molecules ‘cool down’ to 0 °C again.
This activity can be modified to demonstrate the thermal expansion of benzene, with eight students surrounding about 20 ‘benzene’ students with a slack rope, until the pressure through ‘heating’ becomes too strong and the surrounding students are forced to let go of the rope.
The painting An Experiment on a Bird in the Air Pumpw1 by Joseph Wright of Derby (1768) can be used to teach the history of vacuum.
Divide the class into groups of three, and give each group a reprint of the painting and a worksheet showing the silhouette of the main devices and characters (see image on the right; to be downloaded onlinew2). The following three questions tend to promote discussion about the painting:



After the discussion, the whole scene can be re-enacted by the students using a vacuum pump, with a ‘Schokokuss’ (a small chocolate-covered cream cake) to replace the bird.
This activity is useful for introducing the idea of electrons as moving charges that transport energy. One student represents the source of energy (a battery): he or she stands at one end of the classroom and hands out small bags of jelly babies (energy). At the other end of the classroom, another student represents the ‘consumer’ of energy: he or she collects the bags. You can put some tables in the centre of the room to mark out a circuit.
The remaining students represent the electrons: they queue at the energy source and, one by one, are given a bag of jelly babies, then enter the ‘electric circuit’ and walk/run to the ‘consumer’ to hand over the sweets (energy). They then go back to the source to queue again. The ‘electricity’ keeps flowing until the source student runs out of bags (the battery is flat). This activity can be extended to represent parallel and series circuits.
This activity is a useful way to demonstrate that the conductivity of metals decreases with increasing temperature, something that can otherwise only be determined experimentally. Outdoors, use chalk to draw a rectangle of 2 x 5 m to represent the cross-section of a conducting cable. Ask about 20 students to stand inside the rectangle; they represent metal atoms. The remaining 10 students (electrons) try to run through the cable while the atoms either stand still (low temperature) or oscillate by moving their bodies (high temperature). The time it takes for the ‘electrons’ to pass through the ‘cable’ is measured with a stopwatch.

To introduce the topic, ask one student (student 1) to read aloud the story of the discovery of the law of free fallw3. While one of Galileo’s assistants (student 2) marks time by playing regular guitar beats, Galileo himself (student 3) and his second assistant (student 4) carry out their experiments with a table-tennis ball (or similar – a more solid ball gives better results) and an inclined plane with (metal) cross-strings at different heights (see image on the left).
The ball is allowed to roll down the inclined plane from different heights. Student 4 records:
In just one lesson, this method yields excellent material to illustrate the idea of half-life, without any complicated or dangerous experimental apparatus.
A game board of 6 x 6 fields is filled with 36 red gaming pieces. Two easily distinguishable dice are thrown and their numbers are used as the X value (die 1) and the Y value (die 2). The red gaming piece on the field that corresponds to the coordinates (X, Y) on the dice disintegrates into (is replaced by) a blue piece. If a pair of numbers is thrown a second time, nothing happens to the pieces, but the double throw is counted. After each set of 10 double throws, the total number of times that the two dice have been thrown (t) and the number of remaining red gaming pieces (N) are recorded. A graph of t against N is used to determine the half-life.
To illustrate different half-lives, you could use 8-sided dice and a game board with 8 x 8 fields, or change the rules so that each field has to be hit twice before the red pieces disintegrate.
Some of the activities in this article were inspired by the work of others. The author, therefore, would like to acknowledge his debt to Pöpping (2003; Radical polymerisation of ethene to polyethylene), Schreiber (2004; Physical states of water), Fallscheer (2006; Electric circuit), Bührke (2003), Drake (1975), Hepp (2004) and Riess et al. (2005; Galileo’s law of free fall), and Barke & Harsch (2001; Nuclear disintegration and half-life).
This article gives specific and clear ideas about how teachers can use drama to facilitate students’ learning of abstract concepts in physics and chemistry. The activities can be used mainly while tackling standard topics in the curriculum.
The fact that the suggested activities do not need complicated or expensive resources makes them easy to use in the classroom. Teachers are given clear and concise guidelines about how to include creative writing and role playing in their lessons. This not only makes science lessons more interesting and fun for the students, but also means they will feel more involved and responsible for their own learning experience. This will also attract students who are more oriented towards languages and the arts because it stimulates their imagination and creativity. Additionally, it can help teachers to start applying small changes to their teaching, as well as provide new ideas for teachers who have already started implementing similar activities in class.
Catherine Cutajar, Malta
Download this article as a PDF