Cellulose: from trees to treats Understand article
The same molecule that keeps mighty trees standing also led to the first multicellular life forms – and can even be used to make sweet treats.
What is the most abundant large molecule on Earth? Perhaps a synthetic polymer? In fact, we are most likely to come across this molecule in the natural world – walking through a forest, for example. That’s because the molecule is cellulose – the substance produced by plants for structural support.
Earth’s plants produce at least 100 billion (1011) tonnes of cellulose each year – hundreds of times greater than the amount of plastics produced in the same time. As well as being hugely abundant, the cellulose produced by plants is extremely useful. You might be reading this article printed on a piece of cellulose-based paper, for example, and we dress in T-shirts and jeans made of cotton, another example of cellulose. Our furniture at home consists mostly of wood, which itself is mostly cellulose; some of us even live in wooden houses. Many people also use wood as an energy source to heat their homes, perhaps due to the timeless appeal of a real fire. And as a biofuel, wood is a key renewable energy resource.

Joseph / Flickr
What is cellulose?
Despite its giant molecular size, cellulose is a surprisingly simple molecule: it is built up solely from molecules of the sugar glucose. Sometimes several thousand glucose molecules make up a single macromolecule (giant molecule) of cellulose. Glucose itself is made by plants from carbon dioxide and sunlight, via the process of photosynthesis.
A cellulose macromolecule consists of a bundle of individual glucan chains. Each glucan chain is made up of cellobiose molecules, which consist of two linked glucose molecules (figure 1). The linear glucan chains associate with each other through hydrogen bonds, and the large number of these weak linkages gives cellulose its special properties. For example, they help cellulose to exclude water, and thus retain its structural properties even in wet conditions. In addition, they make the molecule resistant to chemical attack by acids and alkalis (Ross et al., 1991).
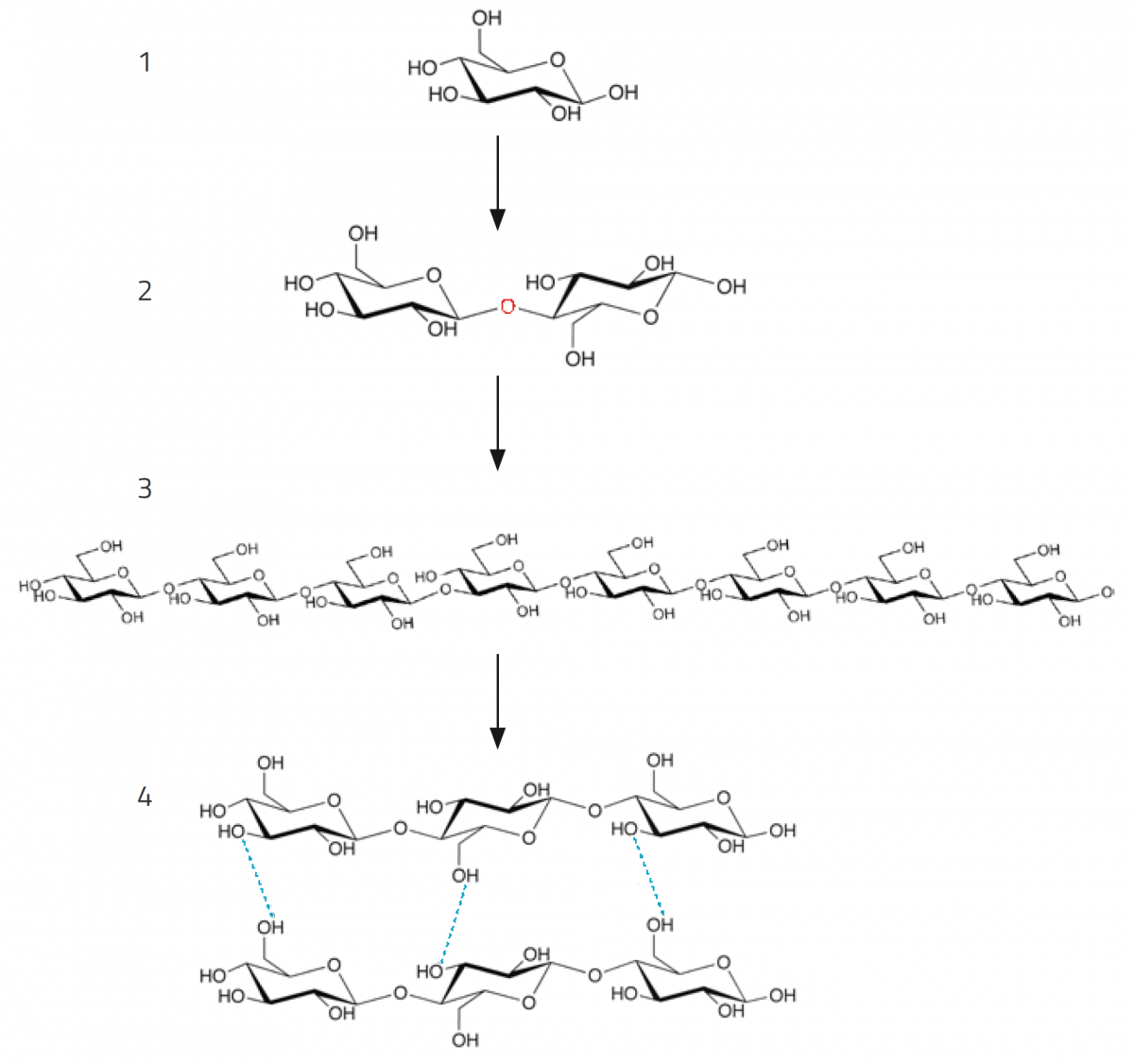
Cellulose: not just a plant thing
These useful properties mean that cellulose is found elsewhere in the living world, not just in plants. Some fungi have a cell wall made of cellulose (although in most fungi, this wall is made from chitin, another abundant macromolecule). Algae, some amoeba and even some invertebrate animals (mostly marine invertebrates known as tunicates) also produce cellulose. In sea squirts, for example, cellulose helps larvae to metamorphose into adults and is also part of the ‘tunic’, a kind of exoskeleton. In social amoeba, these single-celled life forms build up to become multicellular, mushroom-like organisms when nutrients are scarce, with cellulose used in the stalk and spores.
Perhaps surprisingly, some bacteria can also produce cellulose (Ross et al., 1991; Zogaj et al., 2001). Genome sequencing has revealed that this ability is present in a wide range of bacterial species, from the evolutionarily ancient thermophilic bacteria to those that associate with plants or colonise our gastrointestinal tract (Römling & Galperin, 2015). This last group includes two well-known gastrointestinal bugs, E. coli and salmonella (Escherichia coli, Salmonella typhimurium).

Stefan Siebert Photography
So why do bacteria need to produce cellulose? This molecule, which is so strongly associated with rigid structural properties in plants, helps bacteria to adapt to a surprisingly wide range of specialised environments. Cellulose helps bacteria that live in association with plants to attach to the plant’s surfaces – and in the case of pathogenic bacteria, to bind tightly to host cells and cause disease. Some bacteria living in saline springs (including thermophilic species and cyanobacteria) produce cellulose that seems to protect the bacterial cells from desiccation and other environmental threats such as ultraviolet light and even disinfectants.
Cyanobacteria, in fact, may well have been the origin of plants’ ability to produce cellulose. Through evolution, these bacteria became incorporated within plant cells as chloroplasts, bringing with them the genetic information needed to produce cellulose; these genes are now found in the genome of plants (Nobles et al., 2001). But how did this happen? The endosymbiotic theory suggests that, about one billion years ago, free-living photosynthetic cyanobacteria were captured by the ancestors of today’s algae, providing a tremendous advantage to the new combined organisms, which evolved and diversified to form the many photosynthetic species of plants and algae. This theory is supported by the fact that, although today’s chloroplasts have lost most of their original genes, the ancestor gene for cellulose synthase (the enzyme needed to make cellulose) has been transferred to the plant genome and still shows a striking similarity to its counterparts in modern cyanobacteria.
U Römling
Biofilms: making connections
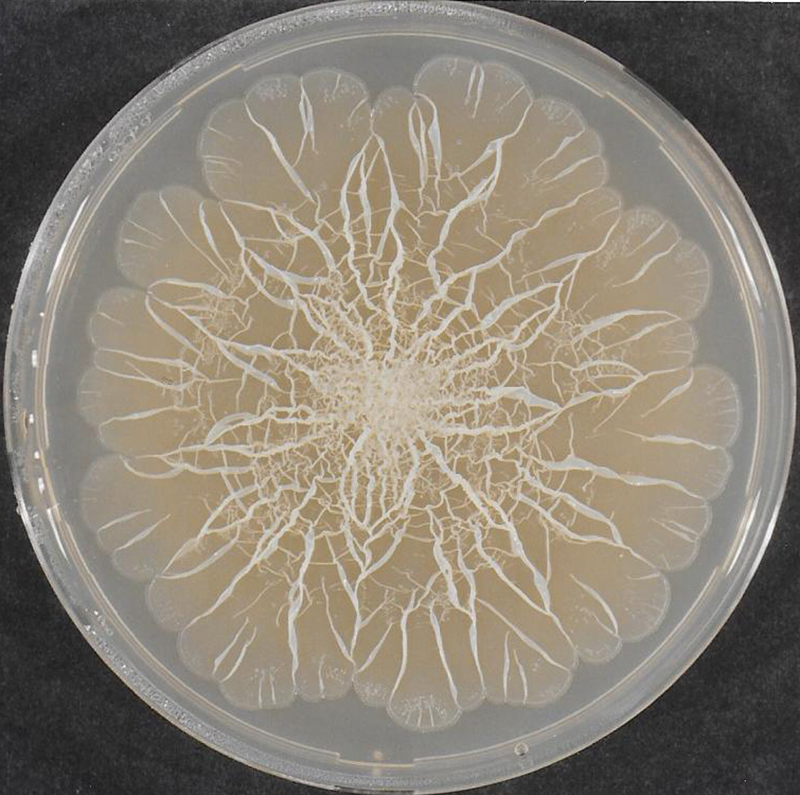
One of the main reasons that bacteria make cellulose is to produce ‘biofilms’ – cellulose films that form a matrix outside bacterial cells and help their multicellular communities to stick together. These bacterial communities were the earliest form of multicellular life on Earth, dating back to around 3.1 billion years ago. Today, there are many micro-organisms that show a similar multicellular lifestyle, which functions remarkably like tissue formation in higher organisms and is thought to make more efficient use of nutrients.
Cellulose films can also help bacteria to interact with higher organisms such as fungi, plants and animals. When highly pathogenic bacteria infect an organism, biofilms provide a mechanism for the bacteria to control the virulence (severity) of the infection. In chronic infections, however, when the bacteria deregulate their biofilm matrix, there is low degree of virulence, allowing the host and bacteria to continue to coexist (Pontes et al., 2015; Ahmad et al., 2016).
Bacterial cellulose: a future supermaterial?

which is becoming popular, is
made using a pellet of
bacterial cellulose.
Römling & Galperin (2015)
Bacterial cellulose has some very special features that make it different from plant cellulose, and these are only just beginning to be explored. Here are some of them: it is especially pure; it has a high surface area and water retention capacity; and it is a natural nanomaterial. Bacterial cellulose has considerable promise in terms of its economic value, and some bacterial cellulose products already being produced commercially. For example:
- ‘Nata de coco’ is a popular, low-calorie sweet from the Philippines. It is made almost completely from bacterial cellulose produced by fermenting coconut milk.
- Kombucha tea, a traditional Asian fermented tea drink, is made using a pellet of bacterial cellulose with a variety of bacteria and yeast strains embedded in it. Promoted as a health drink, it is becoming popular worldwide.
- In medicine, bacterial cellulose is used as a wound dressing, particularly for chronic wounds, because it is mechanically strong and can retain water. As it is also biodegradable and biocompatible, bacterial cellulose has many other potential medical uses: in drug delivery, for example, or in rebuilding damaged tissue, where it can provide a biodegradable, non-immunogenic ‘scaffold’ that living cells adhere to.
This ancient natural material could be well on its way to becoming a supermaterial of the future – revealing another way in which bacteria can help, rather than harm, us.
References
- Ahmad I et al. (2016) BcsZ inhibits biofilm phenotypes and promotes virulence by blocking cellulose production in Salmonella enterica serovar Typhimurium. Microbial Cell Factories 15: 177. doi: 10.1186/s12934-016-0576-6
- Nobles DR, Romanovicz DK, Brown RM Jr. (2001) Cellulose in cyanobacteria. Origin of vascular plant cellulose synthase? Plant Physiology 127: 529-542
- Pontes MH et al. (2015) Salmonella promotes virulence by repressing cellulose production. Proceedings of the National Academy of Sciences of the United States of America 112: 5183-5188. doi: 10.1073/pnas.1500989112
- Römling U, Galperin MY (2015) Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends in Microbiology 23(9): 545-557. doi: 10.1016/j.tim.2015.05.005
- Ross, P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiological Reviews 55: 35-58
- Zogaj X et al. (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produces cellulose as the second component of the extracellular matrix. Molecular Microbiology 39:1452-1463
Resources
- Find out about starch, another macromolecule built up from glucose molecules:
- Cornuéjols D (2010) Starch: a structural mystery. Science in School 14: 22-27.
Review
This article would make excellent background reading, and could be linked to a lesson on the structure and function of biomolecules. The fact that cellulose is produced not just in plant cell walls but also by some bacteria could provoke some discussion and/or research on biofilms, and also on the nature of bacterial virulence. Students could be tasked with researching uses of bacterial cellulose, e.g. for medical use and in foods, and presenting the information to the remainder of the class, or as a small homework project. In addition, some comprehension questions could be set, such as:
- How is cellulose important in tunicates?
- What is the role of cellulose in bacteria that live in saline springs?
- Describe and explain the role of a biofilm.
- What is meant by the term ‘virulence’?
Dr Shelley Goodman, teacher of applied science, UK





