Science under your skin: activities with tattoo inks Teach article
Why not make science relevant to your students’ lives with some simple practical activities using tattoo inks?
In recent years, tattooing has become more and more popular, especially among young people. Why? Fun, a statement of personal identity, and peer pressure are all potential motivations. However, the decision to get a tattoo should not rely simply on social or aesthetic reasons. Other considerations should include health concerns about the ingredients of tattoo inks, the risks of unclean tattooing practices, potential costs for tattoo removal, and the minimum age for getting a tattoo without parental permission.
Behind all the practices of tattooing is a lot of science: from the biochemical effects of tattoo inks to the chemistry of pigments. To make science more relevant to the lives of our students, we have developed a lesson plan for lower-secondary schools that uses hands-on activities related to tattoos (Stuckey et al, 2013; Stuckey & Eilks, 2014; Stuckey & Eilks, 2015). Here we present four of the activities to investigate the contents and thermal stability of tattoo inks.

Detection of metal ions in tattoo inks
Some of the components of tattoo inks, such as heavy metal compounds, can be harmful to health. A simple flame test can be used to indicate the presence of different metals in tattoo inks.
Materials

Image courtesy of the authors
Each group of students will require:
- Wooden splints
- A Bunsen burner
- Crucible tongs
- A selection of tattoo inks (colours and brands)
Procedure
- Dip the end of a splint into a small drop of tattoo ink.
- Using the crucible tongs, hold the splint in the flame of the Bunsen burner.
- Record the colour of the flame. The colour corresponds to particular metal atoms in the ink. For example, blue tattoo inks regularly produce a green flame due to the presence of copper atoms.
- Cut off the used end of the splint or use a new one to repeat the experiment with a different ink sample.
- Suggest which metals are contained in which inks.
Discuss with your students whether there are allergy or safety concerns about these metals. For example, red inks containing chromium salts can often cause allergic reactions.
Identifying the compounds in the inks
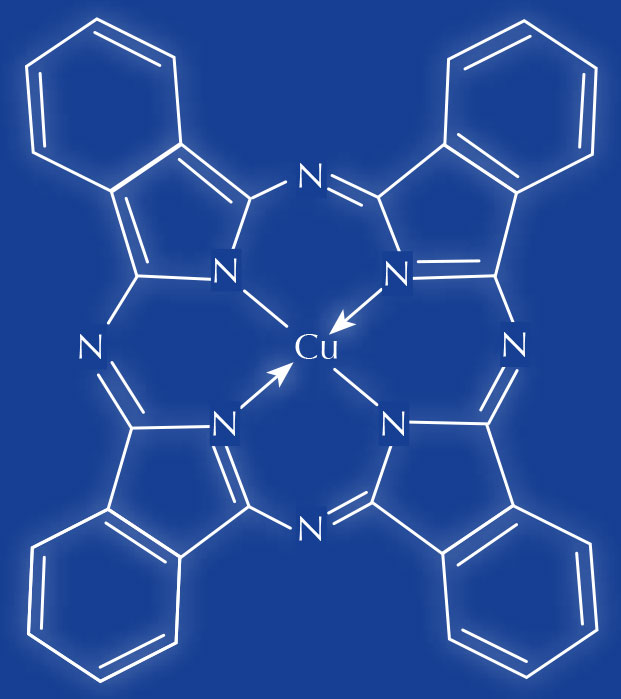
74160)
Image courtesy of Nicola Graf
While there is a European regulatory framework for tattoo chemicals, not all countries have signed up to it. In some countries, such as Germany, tattoo inks must be licenced and comprehensibly labelled. Not all brands of tattoo ink provide this level of detail, however; although they are not licenced for use in Germany, they can be bought cheaply over the Internet. If your tattoo inks are comprehensibly labelled, your students can investigate their contents in more detail.
- Referring to the labels, list the colouring agents in the inks, including the ‘CI’ number.
- Consult Colour Indexw1, an online reference database of dyes and pigments, to determine the identity of the chemicals. For example, CI 74160, which is found in some blue tattoo inks, is the pigment phthalocyanine blue, a copper complex.
- How do these results compare to the results of the flame tests in the previous activity?
- What can you find out about these pigments? For example, are there any health concerns? What other purposes are the pigments used for? Some tattoo pigments, for instance, are also found in car finishes.
If your tattoo inks are not comprehensibly labelled, why might this be a problem?
Stability of tattoo inks
Students can also investigate the thermal stability of different tattoo inks. It is important that the inks are thermally stable to avoid changes once they are tattooed into the skin.
Materials

seconds
Image courtesy of the authors
- Porcelain crucibles with lids
- Crucible tongs
- A tripod
- Metal gauze
- A Bunsen burner
- A selection of tattoo inks
Procedure
- Put a drop of one ink into a porcelain crucible.
- Put a lid over the crucible and place it on the gauze above the Bunsen burner.
- Heat the crucible for 30 seconds, then remove from the heat using the tongs.
- Remove the lid and record any changes in the appearance of the ink drop.
- Repeat for the other inks.
Many tattoo inks are quite heat resistant but others rapidly decompose into a muddy brown mass. When tested by our students, some of the cheap inks bought via the Internet decomposed rapidly. There are also reports of some inks losing their colour when exposed to sunlight.
Investigating the impact on enzyme activity
The potential health impact of tattoo inks can be investigated by looking at their effects on enzyme activity.
Materials
- Beakers
- Petri dishes
- Aqueous hydrogen peroxide (3%)
- Raw potatoes
- A selection of tattoo inks, diluted with water to a thin, water-like, consistency.
Procedure
- Carefully cut a potato into pieces around 1cm3.
- Place one piece of potato into one of the diluted tattoo inks and leave for 10–15 minutes.
- Remove the piece of potato and place it in a Petri dish of hydrogen peroxide solution.
- Observe what happens.
- Repeat for the other tattoo inks.
- Place one piece of potato that has not been treated with ink in the Petri dish full of hydrogen peroxide solution.
Potatoes contain the enzyme catalase, which catalyses the decomposition of hydrogen peroxide into water and oxygen. The piece of potato that was not treated with tattoo ink will react strongly with the hydrogen peroxide solution, causing effervescence as oxygen is generated. The reactions of the treated pieces of potato will vary, as many of the metal ions used in tattoo inks (such as copper) inhibit the action of catalase. - How might the observed effects on the potato pieces translate to the human body? Are the effects cause for concern?
References
- Stuckey M et al (2013) The meaning of ‘relevance’ in science education and its implications for the science curriculum. Studies in Science Education 49: 1–34. doi: 10.1080/03057267.2013.802463
- Stuckey M, Eilks I (2014) Raising motivation in the chemistry classroom by learning about the student-relevant issue of tattooing from a chemistry and societal perspective. Chemistry Education Research and Practice 15: 156–167. doi: 10.1039/C3RP00146F
- Stuckey M, Eilks I (2015) Chemistry under your skin? Experiments with tattoo inks for secondary school chemistry students. Journal of Chemical Education 92: 129–134. doi: 10.1021/ed400804s
- The online supporting information of this article includes instructions for performing the experiments.
Web References
- w1 – The Colour Index is an online reference database of dyes and pigments.
Resources
- German-language instructions for carrying out these and other activities related to tattoos can be downloaded free of charge from the Profiles Bremen website.
- Full teaching and learning materials for the activities (in German) can be found in:
- Stuckey M, Eilks I (2014) Tätowierungen – Chemie, die unter die Haut geht. RAABits Chemie Sekundarstufe I, February: 1-30
Review
Although not yet ubiquitous, tattoos have become more prevalent in recent years and getting tattooed may now be regarded as a rite of passage into adulthood by many school pupils. The activities described by Stuckey and Eilks highlight an additional aspect of tattoos that such pupils need to consider when deciding whether or not to “get inked,” namely the chemical compositions of the inks that are implanted into the skin during tattooing.
For the science teacher, this tattooing context shows the contemporary relevance of methods of chemical analysis and toxicology studies: paper chromatography and thin layer chromatography may be carried out in school science laboratories, provided that the solvents required are common. Questions about the regulation of consumer chemicals are also raised, and could be a topic for research by pupils. Furthermore, these investigations provide an opportunity to enter into discussions about the personal, legal and health issues associated with tattooing, which may also lead into discussions about self-image, peer pressure and decision making. A science teacher considering carrying out these investigations would be well advised to speak to/involve colleagues involved in their school’s personal, social, health and citizenship education programme, (or equivalent) in order to gain some insight into the pastoral issues that could arise.
Matthew Fletcher, Kingswood School, UK
