Hydrogen: the green energy carrier of the future? Understand article
Hydrogen may be the fuel of the future, but how can we produce it sustainably? Karin Willquist explains.

iStockphoto
Hydrogen has been called ‘the energy carrier of the future’ – because it can be oxidised in a fuel cell to generate electricity, for example to power cars, without releasing carbon dioxide (CO2), and it can be produced in remote places without an electricity infrastructure. In contrast to available resources such as natural gas and gasoline, hydrogen has to be produced, making it an energy carrier and not a fuel.
An energy system in which hydrogen is used to deliver energy – a hydrogen economy – was proposed by John Bockris in 1970; in 1977, an international hydrogen implementing agreement was established to work towards itw1.
Hydrogen is mainly used now as a chemical reagent rather than an energy carrier, but there is no doubt that it has the potential to transform our transport and energy systems. However, realising its potential is not easy. Most fuels currently in use are liquids, solids or gases with high energy per volume (energy density). Hydrogen, in contrast, has a low energy density: at a given pressure, burning one litre of hydrogen produces one third of the energy that burning a litre of methane does. This poses problems of storage, distribution and use that are being addressed by scientists (Schlapbach & Züttel, 2001)w2. A more fundamental challenge, however, is that of producing hydrogen in a sustainable manner. This is what I shall focus on here.
Ways to produce hydrogen

powered by hydrogen fuel
cells
Image courtesy of Felix O;
image source: Flickr
Hydrogen is an abundant element on Earth’s surface, normally linked to carbon in carbohydrates (in plants) or to oxygen in water (H2O). Hydrogen gas (H2), in contrast, exists only in small quantities on Earth. One of the challenges for sustainable hydrogen production is releasing H2 from its bonds with carbon and oxygen.
Currently, H2 is produced mainly from fossil fuels (e.g. natural gas) by steam reforming: heating the fuels to high temperatures with waterw2:
CH4 + H2O → CO + 3H2 (1)
CO + H2O → CO2 + H2 (2)
However, this method relies on fossil fuels and releases CO2 , causing the same emission problems as burning fossil fuels. Steam reforming is only sustainable if renewable hydrocarbons such as biogas are used, because the CO2 released has previously been absorbed in the production of the hydrocarbons.
H2 can also be produced by electrolysisw2, whereby electricity is used to split H2O into H2 and oxygen:
2H2O → 2H2 + O2 (3)

charger from Powertrekk.
Just add some water, and
after a few minutes you have
a battery for your mobile
phone
Image courtesy of David
Berkowitz; image source: Flickr
This method can be sustainable if the electricity is from renewable resources such as wind, wave or solar power. H2 can thus be used to store energy on windy days when the windmills produce more electricity than can be consumed.
Interestingly, H2O splitting occurs naturally in the oceans, because microscopic algae and cyanobacteria use solar energy to split water in a process called biophotolysis (Equation 3). However, the rate of H2 production is extremely slow.
Efforts have been made to increase the production rate under controlled conditions using modified micro-organisms, but the processes are still too slow and expensive to be a realistic source of H2 any time soon (Hallenbeck & Ghosh, 2009).
Finally, biohydrogen can be produced from crops and from industrial, forestry and agricultural waste, using bacteria. Like us, these bacteria oxidise plant material as a source of energy, but unlike us, they live in anaerobic environments (lacking oxygen). In aerobic respiration, we use O2 to oxidise sugars, e.g.
C6H12O6 + 6O2 → 6CO2 + 6 H2O (4)
In contrast, to oxidise the substrate as far as possible and thus optimise their energy gain, these anaerobic bacteria reduce protons, released during substrate oxidation, to H2 (Equation 6, below).
Hot bugs

C. saccharolyticusbacteria
under the electron
microscope
Image courtesy of Harald
Kirsebom
During my PhD, I investigated the hydrogen-producing abilities of one of these bacteria, Caldicellulosiruptor saccharolyticus (Figure 1), which lives in hot springs: anaerobic environments at 70°C, with low levels of available carbohydrates. This bacterium is of particular interest because it is twice as efficient as most bacteria used for H2 production.
Unlike humans, C. saccharolyticus gains energy from a wide spectrum of plant building blocks: not only glucose, but also, for example, xylose (Willquist et al., 2010).
This allows the bacteria to produce H2 from waste such as that produced during potato, sugar and carrot processing, as well as from industrial waste from pulp and paper production, or agricultural waste such as straw.
This is a promising start, but even C. saccharolyticus releases only 33% of the potential H2 that could be released from the substrate. Equation 5 shows the potential complete oxidation of glucose, releasing 12H2 per molecule of glucose. Equation 6 shows the dark fermentation performed by C. saccharolyticus, which releases only 4H2 (33%) per molecule of glucose. The rest of the energy is released as acetate (CH3COOH).
Total conversion of glucose to H2: C6H12O6 + 6H2O → 12H2 + 6CO2 (5)
Dark fermentation: C6H12O6 + 2H2O → 4H2 + 2CO2 + 2CH3COOH (6)
To release the rest of the H2 from the acetate requires external energy. Alternatively, methane (CH4) – which can be steam reformed to release H2 (Equations 1 and 2) – can be generated from acetate. Luckily, there are three promising ways of doing this (Figure 2).
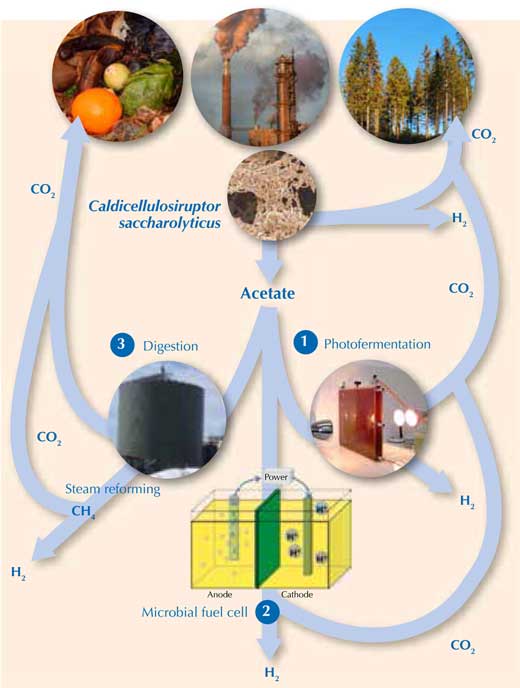
Images courtesy of Holger / pixelio.de (waste), Michael Cavén (paper factory), Keith Bryant (trees), Marcel Verhaart (C. saccharolyticus), Jakub Gebicki (photobioreactor), Gokce Avcioglu, METU Biohydrogen Research Lab, Turkey (anaerobic digestion reactor) and Karin Willquist (microbial fuel cell)
- Using sunlight to convert acetate to H2 with photofermentative bacteria (Equation 7)w3. However, like algal H2 production, this process is currently too slow and expensive to be commercially viable in the near future (Hallenbeck & Ghosh, 2009). 2CH3COOH + 4H2O → 8H2 + 4CO2 (7)
- Using electricity to push the reaction of acetate to H2 in a microbial fuel cell with a mixture of bacterial species (Equation 7)w4. This is an elegant concept, but its application is currently limited by low production rates (Hallenbeck & Gush, 2009). (To learn how to build your own microbial fuel cell, see Madden, 2010.)
- Using methane producers (Archaea) to digest the acetate, generating methane (Equation 8). The combination of dark fermentation (Equation 6) and methane production is known as the hythane process (hydrogen + methane), and can convert approximately 90% of the original substrate to H2 and methane. CH3COOH → CH4 + CO2 (8)The methane can then be steam reformed releasing H2.

powered by a hydrogen
fuel cell
Image courtesy of Bull-Doser;
image source: Wikimedia
Commons
To put the hythane process into perspective: if four people in a house eat 10 kg potato products each in one month, their waste could fuel 0.5% of their monthly domestic energy requirement (3500 kWh), provided that the H2 produced is used directly (to avoid energy losses) and that the house is equipped with a heat and power fuel cellw5. More hydrogen could of course be generated from other waste – 0.5% is just from potatoes.
This is a rough estimate of the potential of the hythane process, based on a) 30% energy loss in the production of H2 and CH4 (hythane) and b) 30% in then steam reforming CH4 to H2. The steam-reforming step (b) is used in the production of hydrogen from natural gas, and is a well developed commercial technique. The production of hythane (a), however, is not yet that efficient, although research is ongoing to improve the efficiency to reach 70% (as in the example) and thus make the production of biohydrogen competitive with the steam reforming of fossil fuels for producing hydrogen.
Although there has been some recent progressw6 (see box), it is too early to give a reliable time estimate for when sustainable H2 production could play a significant part in supplying us with energy. However, as poet Mark Strand once said, “The future is always beginning now.”
Research into hydrogen storage and production

Storing hydrogen safely and efficiently is one of the main technological challenges to adopting hydrogen as an energy carrier. The Institut Laue-Langevin (ILL)w7 has firmly established itself in frontier research into the hydrogen economy, using neutron diffraction to monitor hydrogenation and dehydrogenation reactions in potential hydrogen storage materials. To find out more, visit the ILL websitew7.
The powerful X-ray beams of the European Synchrotron Radiation Facility (ESRF)w8 have recently probed the complex mechanisms by which hydrogen is produced by enzymes called hydrogenases. Most of these enzymes work under anaerobic conditions and are, in fact, inhibited by the presence of oxygen. Hydrogenases that remain active under aerobic conditions, therefore, are of great interest for technologies such as enzymatic fuel cells and the light-driven production of hydrogen. A German team of scientists has recently solved the crystalline structure of one of these enzymes (Fritsch et al., 2011) – perhaps a step towards a hydrogen economy?
Both ILL and ESRF are members of EIROforumw9, the publisher of Science in School.
References
- Fritsch J et al. (2011) The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre. Nature 479: 249–252. doi: 10.1038/nature10505
- Download the article free of charge here, or subscribe to Naturetoday: www.nature.com/subscribe
- Hallenbeck P, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends in Biotechnology 27: 287–297. doi: 10.1016/j.tibtech.2009.02.004
- Madden D (2010) The microbial fuel cell: electricity from yeast. Science in School14: 32-35. www.scienceinschool.org/2010/issue14/fuelcell
- Rifkin J (2002) The Hydrogen Economy: the Creation of the Worldwide Energy Web and the Redistribution of Power on Earth. New York, NY, USA: JP Tarker. ISBN: 1585421936
- Schlapbach L, Züttel A (2001) Hydrogen-storage materials for mobile applications. Nature 414(6861): 353–358. doi: 10.1038/35104634
- Download the article free of charge here, or subscribe to Naturetoday: www.nature.com/subscribe
- Willquist K, Zeidan A, van Niel E (2010) Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: an efficient hydrogen cell factory Microbial Cell Factories 9: 89. doi: 10.1186/1475-2859-9-89
- Microbial Cell Factories is an open-access journal, so the article is freely available.
Web References
- w1 – To learn more about the hydrogen implementing agreement of the International Energy Agency, see: http://ieahia.org
- w2 –To learn more about hydrogen prospects, see Joseph Romm’s analysis on the Environmentalists for Nuclear Energy website (www.ecolo.org; under ‘documents’) or via the direct link: http://tinyurl.com/77dhx8x
- See also Joan Ogden’s peer-reviewed analysis Hydrogen as an Energy Carrier: Outlook for 2010, 2030 and 2050 on the website of the University of California: http://escholarship.org/uc/item/9563t9tc
- w3 – For a video about how hydrogen is released from potato biomass using sunlight, see: www.biohydrogen.nl/hyvolution
- w4 – To learn more about microbial fuel cells, see: www.microbialfuelcell.org
- w5 – To find out more about heat and power fuel cells, see: www.fchea.org/index.php?id=57
- w6 – To read about recent progress on a biohydrogen fuel station in Taiwan, see the Focus Taiwan website (http://focustaiwan.tw) or use the direct link: http://tinyurl.com/7jao2tp
- w7 – ILL is an international research centre at the leading edge of neutron science and technology, based in Grenoble, France. To learn more, see: www.ill.eu
- For more information on ILL’s research into the hydrogen economy, see the ILL website or use the direct URL: http://tinyurl.com/illhydrogen
- w8 – Situated on the same campus as ILL, in Grenoble, France, ESRF operates the most powerful synchrotron radiation source in Europe. To learn more, see: www.esrf.eu
- For more information on ESRF’s research into hydrogen storage, see the ESRF website or use the direct URL: http://tinyurl.com/87bnj4c
- w9 – To find out more about EIROforum, see: www.eiroforum.org
Review
Following the publication of Jeremy Rifkin’s book on the hydrogen economy (2002), this topic is frequently addressed in media as a real possibility for the near future. Another common issue surrounding hydrogen is its supposed role as a clean energy source. In this article, Karen Willquist offers a thorough overview of the issues involved in hydrogen production and of the ongoing research – including her own work – into sustainable ways to achieve this goal.
Given the author’s clear approach, the article is particularly suitable for science teachers and upper-secondary-school students (ages 14-19) wishing to deepen their knowledge of this complex topic. Furthermore, both teachers and students will benefit from the many resources listed.
The article would be relevant for lessons on biochemistry (respiration, fermentation and photosynthesis), physics (fuel cells, thermodynamics: energy and efficiency), environmental science (energy resources, fossil fuels and renewable resources), biology (algae, bacteria, cyanobacteria and Archaea) and organic chemistry (hydrocarbons and steam reforming). It could also provide valuable background reading before a visit to a power plant or research laboratory working on fuel cells or hydrogen production, use or storage.
The article could be used to initiate a discussion on the difference between energy resources and energy carriers; the problems of hydrogen use and storage; and possible scenarios for the transition from our hydrocarbon economy to a hydrogen economy.
Suitable comprehension questions include:
- Which of the following apply to respiration, to dark fermentation, or to both?
- The presence of glucose
- The presence of oxygen
- The absence of oxygen
- Which of the following is not a process involved in converting acetate into hydrogen?
- Dark fermentation
- The use of electricity in a microbial fuel cell
- The hythane process
Giulia Realdon, Italy





