Melts in your viscometer, not in your hand Teach article
Teaching viscosity can be sweetened by using chocolate.

Chocolate is one of the only foodstuffs that stays solid at room temperature but easily melts at body temperature. This peculiar behaviour is due to cocoa butter, a fatty substance obtained from the seeds of cacao, which is solid under 25 °C but liquid at 37 °C.
As thousands of children around the world could assure you, chocolate quality is an important issue. When chocolate is liquid, its quality is determined mainly by its viscosity. In this article we present a method, designed by our students, of measuring chocolate viscosity using a viscometer constructed from simple and easily obtainable materials.
After building the apparatus, which should take 2–3 hours, you can use it to measure the viscosity of water, syrup, honey and chocolate, and compare the values with published data.
Viscosity
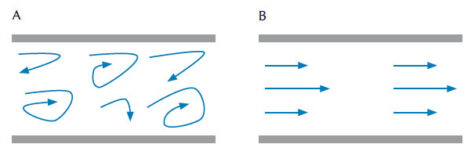
The viscosity of liquids and gases is defined as the material’s resistance to deformation under stress, which is determined by the friction between particles in the material. The thicker a material is, the greater its viscosity. According to Poiseuille’s Law, the viscosity factor of a fluid that flows out of a syringe nozzle (meaning that the flow of liquid is laminar instead of turbulent, figure 1) is calculated as:
n = πr4ρt / 8V
where:
n is the viscosity factor
r is the radius of the syringe’s nozzle
ρ is the special weight of the fluid, where ρ = dg, d is the density of the fluid (d = m / V) and g is the acceleration due to gravity (9.8 ms-2)
t is the time required for the fluid to be emptied from the syringe
V is the volume of the fluid (in our experiments, we used 60 ml for all fluids).
The viscosity is measured with a special instrument called a viscometer. The unit of measurement of viscosity is the Poiseuille (Pl), equivalent to a Pascal second, 1 Pa s = 1 N m–2 s = 1 kg m−1 s−1. The unit poise (P) = 0.1 Pa s is also used.
Constructing a viscometer
The following experiment outlines how to build a viscometer (figure 3) using a method that we developed with our students. The central idea was proposed by the students, who asked, given Poiseuille’s law, how can we build a viscometer using everyday materials? The device in question had to be able to measure viscosity at different temperatures. We chose to develop a flow-cup type of viscometer, because the dark colour of chocolate makes a ball-falling type of viscometer – which measures the time taken for a ball of known volume to fall through a liquid – completely unsuitable.

experimental design showing
the thermometer in the water
bath (A), the thermometer in
the syringe (B), and the
styrofoam insulation (C).
Materials
- One 60 ml syringe with a 2.5 cm long nozzle
- An empty shampoo bottle, 7 cm in diameter and longer than the syringe
- Insulating polystyrene material
- Self-adhesive aluminium sheets
- Two thermometers capable of measuring between 0 and 100 °C
- A method for securely supporting the apparatus above the collection flask / beaker. This can be a frame designed and assembled by the students, an arrangement of stands and clamps, or simply two stacks of books.
- Craft knife or scissors
- Sticky tape
- A flask / beaker with a volume scale on the outside
- Pan balance capable of measuring 0.1 g increments
- Modelling clay, e.g. Plasticine®
- Calipers or a ruler.
Procedure
- Measure the internal size of the syringe nozzle using calipers or a ruler, or note the manufacturer’s description.
- Remove the lid from the shampoo bottle.
- Use the knife or scissors to remove the base of the shampoo bottle.
- Invert the bottle so the neck is pointing down.
- Insert the syringe, with the nozzle down, into the neck of the shampoo bottle.
- Seal the syringe nozzle and the space between the syringe nozzle and shampoo bottle with modelling clay.
- Cut the insulating foam to produce three pieces each measuring 30 cm x 30 cm x 5 cm.
- Place the long edges of the three foam pieces together to make a triangular tube and tape together.
- Place the syringe-bottle construction inside the insulating foam tube.
- Wrap the construction in aluminium.
- Cut a small piece of insulating foam to fit over the top of the syringe-bottle construction and make two holes for the thermometers, so that one thermometer goes into the syringe and one sits between the wall of the syringe and the shampoo bottle.
- Insert the thermometers through the two holes in the foam (figure 2).
- The whole experimental device should then be placed on the base so that the nozzle of the syringe points vertically down.
- Place the flask / beaker on the pan balance and set the balance to zero.
- Place the balance under the experimental device so that the syringe points into the flask / beaker.
Using the viscometer

The actual construction of the viscometer by the students is already a valuable piece of experiential learning. In the next step, the students encounter important research questions concerning the role of water in the shampoo bottle as a water bath and the necessity of using a pair of thermometers to make sure that thermal equilibrium between the measured liquid and the water bath is reached.
Materials
- Viscometer apparatus, as described above
- Another 60 ml syringe with a 2.5 cm long nozzle (identical to the one used in the viscometer apparatus)
- Pan balance
- Water (enough to fill the shampoo bottle)
- Kettle or other water heater
- Glass beakers (one for each liquid to be studied)
- Stopwatch or mobile phone with timer function
- Different liquids to be studied; water, honey, syrup, milk chocolate and dark chocolate can all be studied. Experimenting with white chocolate should not be attempted as the emulsifiers used can cause clotting, which stops the liquid chocolate from flowing.
Procedure
- Heat the water and pour it into the shampoo bottle in the viscometer.
- Weigh the empty extra syringe using the pan balance.
- Heat the liquid to be studied.
- Put 60 ml of the heated liquid into the extra syringe.
- Weigh the filled syringe.
- Subtract the weight of the empty syringe from the weight of the filled syringe to determine the fluid’s mass. Taking into account the fluid’s volume, you can then estimate the fluid’s density using the equation d = m / V.
- Use the fluid’s density to calculate the special weight of the fluid, ρ.
- After checking that the syringe is sealed into the apparatus, transfer the heated liquid into this syringe.
- Place the foam lid over the top of the viscometer and wait until the temperatures of the water bath and the heated liquid are the same.
- Remove the modelling clay from the syringe nozzle so that the liquid can start flowing. Start the timer.
- The liquid will flow into the flask below the syringe and the pan balance will measure the mass of the escaped liquid.
- Use the pan balance to determine when all the liquid has left the viscometer. When this has happened, stop the timer.
- Record the time taken for the liquid to flow through the viscometer.
- The viscosity factor can then be calculated using Poiseuille’s law.
Experimental measurements and calculations
Experiment 1
Students can measure the viscosity values for different materials at two different temperatures (20 °C and 80 °C) and rank the liquids in order of increasing viscosity. You can discuss what might cause differences in viscosity between the liquids.
Our students’ results are shown in figures 4 and 5, and tables 1 and 2.
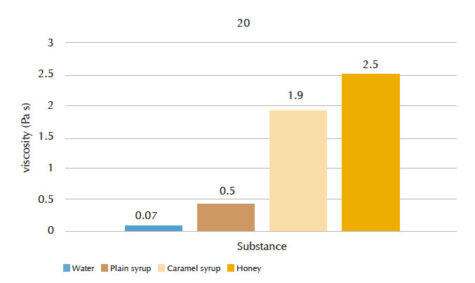
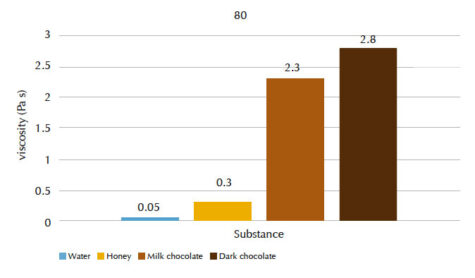
| Substance | Flow time t (s) | Viscosity n (Pa s) |
|---|---|---|
| Water | 4.2 | 0.07 |
| Plain syrup | 6.3 | 0.5 |
| Caramel syrup | 23.5 | 1.9 |
| Honey | 32.5 | 2.5 |
| Substance | Flow time t (s) | Viscosity n (Pa s) |
|---|---|---|
| Water | 2.3 | 0.05 |
| Honey | 4.5 | 0.3 |
| Milk chocolate | 38.0 | 2.3 |
| Dark chocolate | 43.0 | 2.8 |
Experiment 2
Ask your students to measure the viscosity values of chocolate, honey and water at five or more temperatures to study the change in viscosity in relation to temperature.
The time required for water, honey and chocolate to pass through the viscometer at different temperatures, along with their calculated viscosity values, are shown in tables 3, 4 and 5, respectively. In figure 6, changes in viscosity as a function of temperature can be seen for these fluids.
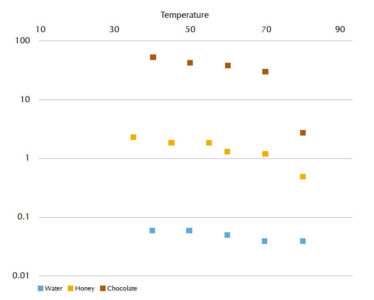
| Temperature Θ (°C) | Flow time t (s) | Viscosity n (Pa s) |
|---|---|---|
| 30 | 4.207 | 0.07 |
| 40 | 3.92 | 0.06 |
| 50 | 3.5 | 0.06 |
| 60 | 3.05 | 0.05 |
| 70 | 2.73 | 0.04 |
| 80 | 2.3 | 0.04 |
| Temperature Θ (°C) | Flow time t (s) | Viscosity n (Pa s) |
|---|---|---|
| 35 | 30 | 2.3 |
| 45 | 24.38 | 1.9 |
| 50 | 17.5 | 1.3 |
| 55 | 15.5 | 1.2 |
| 60 | 7.1 | 0.5 |
| Temperature Θ (°C) | Flow time t (s) | Viscosity n (Pa s) |
|---|---|---|
| 40 | 800 | 52.6 |
| 50 | 660 | 43.4 |
| 60 | 590 | 38.8 |
| 70 | 480 | 31.6 |
| 80 | 43 | 2.8 |
Further questions
To extend the activity, you can ask your students additional questions such as:
- What is the range of the viscosity values for different materials at 20 °C and 80 °C? Why does dark chocolate appear to have a higher viscosity than the other substances?
- What can you conclude about the dependence of viscosity on temperature?
- Does chocolate or honey have a higher viscosity at 80 °C? Try to document your answer based on further bibliographic research.
- Do the viscosity values that you found experimentally for water, honey and chocolate agree with values that appear in your bibliographic research materials? If there are any discrepancies, can you provide any explanations?
The viscosity of chocolate
Molten chocolate represents a dense blend of phospholipid-coated sucrose and cocoa particles in liquid fat. Because of this, the viscosity of chocolate has a complex pattern that is described as non-Newtonian. A particular force is required for chocolate to begin flowing; once the fluid begins flowing, as this force increases, the fluid thins.
Essentially there are two parameters that describe how chocolate flows. The first one is the limit in elasticity, the force that chocolate needs to begin flowing. The second parameter is the plastic viscosity, which is related to the energy that the chocolate requires in order to remain in motion at a constant rate (Beckett, 2000).
Understanding how chocolate flows is not just an interesting lesson for students but also of course very important for chocolate manufacturers.
Acknowledgements
We express our deepest gratitude to our students, Zoe Efthimiadou, Viktoria Kelanastasi and Aggeliki Kosma, for their conscientiousness, bright ideas and hard work.
We also express our thanks to Professors KG Efthimiadis, H Polatoglou and K Melidis from the Physics Department at Aristotle University, Thessaloniki, Greece, for their useful suggestions.
Finally we express our deepest appreciation to Mr N Kyriakides, a school parent who undertook the task of constructing the metal base upon which the whole apparatus was built.
References
- Beckett ST (2000) The Science of Chocolate. London, UK: Royal Society of Chemistry. ISBN: 9780854046003
Review
Science teachers are always looking for new and innovative ways to engage students in learning about our world. In this case, it’s a world of tasty food. Using popular food – chocolate and honey – for physics education is always of interest. This experiment also encourages problem solving, because the equipment is constructed by the students.
Since food is traditionally an integrated topic between home economics, biology, chemistry and physics curricula, this article could give teachers some ideas for collaboration between these subjects. Design and technology classes could also be involved due to the construction of the equipment.
Dr Ingela Bursjöö, Johanneberg School and University of Gothenburg, Sweden





