From gaming to cutting-edge biology: AI and the protein folding problem
How can AI systems like those developed to beat humans at games help unlock the secrets of protein…

Scientists use intense X-ray pulses from the European XFEL to take snapshots of exploding molecules. This can reveal details of how molecules are put together and how they interact with light.
Taking a picture by exploding the subject matter? An international research team at the European XFEL uses this extreme method to take pictures of complex molecules. European XFEL is the world’s largest X-ray free-electron laser (XFEL), which produces high-quality X-rays for scientific research. In one experiment, the scientists used the ultra-bright X-ray flashes to take snapshots of gas-phase iodopyridine molecules.[1] The X-ray laser caused the molecules to explode, and an atomic-resolution image was reconstructed from the pieces.

This method of exploding molecules is particularly useful to investigate molecules with covalent bonds. Chemists often speak of three types of chemical bonds: ionic, covalent, and metallic. Covalent bonds occur in molecules that consist of multiple atoms. These molecules have specific structures and properties and can occur in more than one state of matter: for example, H2O can exist as solid ice, liquid water, or gaseous steam. The component atoms cannot be separated by physical processes, only by a chemical reaction.
In covalent bonds, electrons are shared between two atomic nuclei. Both nuclei attract the same electrons at the same time, which pulls the atoms together to create a covalent bond. The more electrons involved, the stronger the bond. In the classroom we often model the atoms and bonds with balls and sticks, but in reality, they are more dynamic and can rotate and vibrate. When they interact with electromagnetic radiation, like light or X-rays, the electrons in a molecule can change their energy levels. Chemists and physicists investigate these properties to find out more about molecules and their properties.
Molecular physicists study molecules and their movements (also called dynamics). They are interested in the structure of molecules and how they interact with electromagnetic radiation, such as X-rays. One method they use to do this is called the Coulomb explosion; to understand this, we need to be familiar with Coulomb’s law. Remember that like charges repel and unlike charges attract one another. Coulomb’s law states that the attracting or repelling electrostatic force depends on both the magnitude of the charges and the distance between them.
Coulomb’s law is used to calculate the force between electrically charged particles, including atomic nuclei and electrons. In a molecule, the heavy nuclei move less than the lighter electrons. Molecules both rotate and vibrate due to these motions. Molecular physics experiments investigate both the shape, size, and energy levels of molecules and how they ionize or break apart.
At European XFEL, molecular physicists use Coulomb explosions to investigate small molecules. A high-intensity and ultra-short X-ray laser pulse knocks a large number of electrons out of the molecule. Due to the strong electrostatic repulsion between the remaining, positively charged atoms, the molecule explodes within a few femtoseconds – a millionth of a billionth of a second. The individual ionized fragments fly apart and are registered by a detector. The location and time of impact of the fragments are determined and then used to reconstruct their momentum – the product of mass and velocity – with which the ions hit the detector.
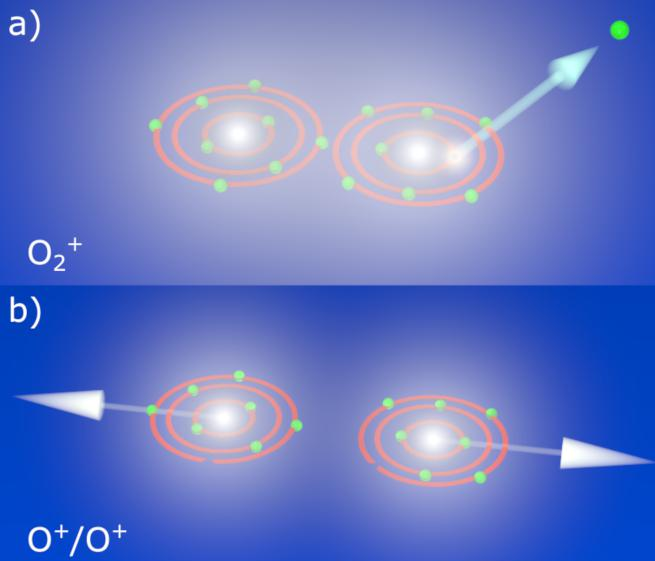
This information can be used to obtain details about the molecule, and with the help of models, scientists can reconstruct the course of reactions and processes involved. You could compare this with investigating a road accident: if you know the final positions of the vehicles, you can infer details of how the accident took place. In science, the ultimate goal of the reconstruction is to record the Coulomb explosion as a molecular movie.[1]
Making molecular movies that record molecules moving or reacting is one of the key goals of free-electron lasers. At European XFEL near Hamburg, ultra-short, ultra-bright X-ray pulses make it possible to create stop-motion films of molecules in action. To take a snapshot of a fast-moving object, you need a short exposure time. Ultra-short X-ray flashes can capture images of the extremely fast movements of molecules. Scientists use many images taken at different times to create animations of how molecules move – often described as a molecular movie.
Scientists at the small quantum systems (SQS) instrument are particularly interested in molecular physics. They observe the structure of molecules over time by recording molecular movies. What does imaging the structure of a molecule mean, exactly? Resolving the overall shape? Measuring all the bond lengths and angles? Seeing an atom move? Researchers are interested in all of these aspects, but they can be hard to measure.[3]
Small gas-phase single molecules, and particularly light atoms like hydrogen, are hard to film, even at an XFEL facility. Most methods for imaging molecules rely on the assumption that imaging takes place before the sample is destroyed. Coulomb explosion imaging is an exception to this rule because it makes use of the destruction process to create the images.
The earliest Coulomb explosion experiments were not suitable for small gas-phase molecules because it was difficult to destroy the molecule and take its picture without deforming it first. However, free-electron laser facilities like European XFEL can produce very brilliant, very short X-ray pulses that make it possible to explode a molecule without deforming its shape.
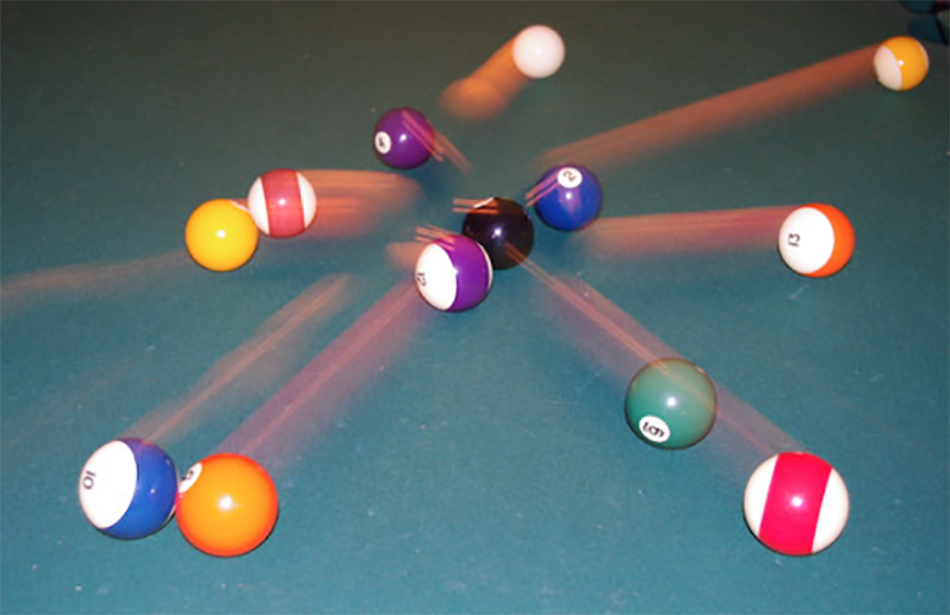
Let us imagine the exploding molecule experiment as a game of billiards. The billiard balls arranged in a triangle at the beginning of the game represent the atoms arranged in a molecule with a specific shape. When they are hit by the cue ball – the X-ray pulse – they fly in different directions and at different speeds. As a physicist, you can measure the momentum of the balls to build a model of the game. If you already know how the cue ball hit, you can use the momentum data from the balls to reconstruct their starting positions.
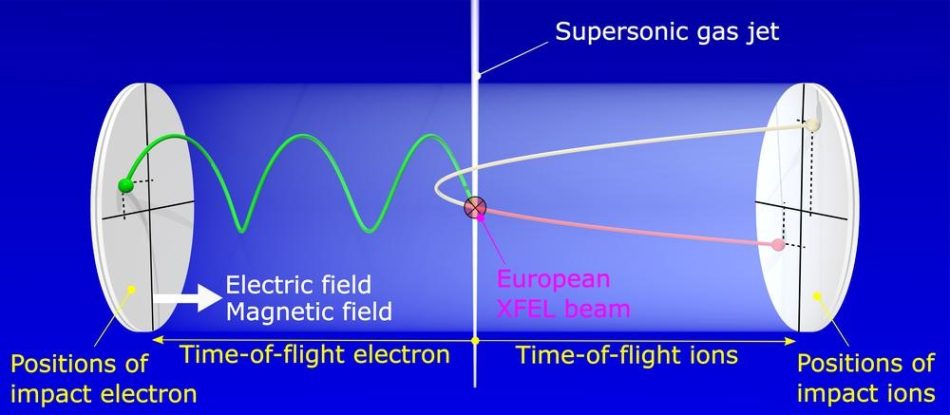
The equipment physicists use to play ‘molecular billiards’ is called the reaction microscope (REMI), and it is part of the SQS instrument at European XFEL. The REMI employs static electric and magnetic fields to guide ionic particles and electrons towards time- and position-sensitive detectors on opposite sides of a spectrometer. From the recorded momenta (speed and direction) of the particles, their emission directions, energies, and relative angles can be retrieved.[4] This information is used to reconstruct the structure of the molecule at the time it exploded – just like the positions of billiard balls before being hit by the cue ball.
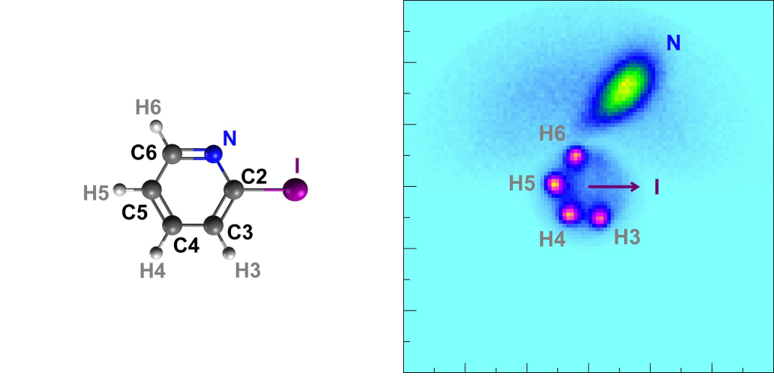
Coulomb explosion experiments make use of the very intense and very short X-ray pulses available at European XFEL for studying processes where several photons are absorbed by a single atom or molecule. This leads to very extreme conditions and provides insights to the fundamental interaction of such pulses with single atoms or small molecules. These experiments are a big step towards imaging small molecules and are still being developed.
Following the first successful demonstrations, the scientific community is keen to use this method to study molecular dynamics in a time-resolved manner. By combining a series of detailed snapshots of a molecule’s structure at different time intervals during a reaction, they hope to create stop-motion animations of atomic movements.
Molecular movies are expected to stimulate developments in various fields of research, and are particularly promising for investigating photochemical processes. These light-triggered chemical reactions are of great importance both in the laboratory and in nature, such as in photosynthesis and in visual processes in the eye. This fundamental research could help develop new ideas for medicine, sustainable energy production, and materials research.
[1] Molecule snapshot by explosion: https://www.xfel.eu/news_and_events/news/index_eng.html?openDirectAnchor=1945
[2] Kastirke G et al. (2020) Photoelectron Diffraction Imaging of a Molecular Breakup Using an X-Ray Free-Electron Laser. Phys. Rev. X 10: 021052. doi: 10.1103/PhysRevX.10.021052
[3] Snapshots of exploding oxygen: https://www.xfel.eu/news_and_events/news/index_eng.html?openDirectAnchor=1784
[4] User meeting presentation: https://www.xfel.eu/users/user_events/users__meetings/2020_users__meeting/slides/index_eng.html
‘Explosive imaging’ is an excellent article that describes a novel technique for working out molecular structure. Judicious use of comprehension questions could compel closer reading of the text. It could be used as a resource to augment discussions of electrostatics and it even offers an alternative application of electrostatic repulsion. It could even be used for the basis of calculations.
It might also lead to comparisons with other physical systems where moving objects allow scientists to make inferences. For example, the Hubble recession of galaxies allows us to infer that our universe began as a Big Bang about 14 billion years ago. Detecting the debris from high-energy particle collisions in the Large Hadron Collider (CERN) allows scientists to make deductions about the structure of sub-atomic particles (amongst other things).
Accident investigators can use the disposition of vehicles or other objects and the state of the environment to determine how or what was at fault for an accident.
Additional comprehension questions:
1. What is a covalent bond?
– In covalent bonds, electrons are shared between two atomic nuclei.
2. Explain how a Coulomb explosion happens.
– A high-intensity and ultra-short X-ray laser pulse removes a lot of the electrons from the molecule. The positive nuclei then repel electrostatically.
3. How do scientists use the ‘Coulomb explosion’ to work out the original molecular structure?
– Detectors record the momentum (= mass x velocity) of the ionised fragments when they hit a detector and use this to work out their original positions.
4. Explain why very short X-ray pulses do not deform the molecule?
– The pulse is so short (and energetic) that the molecule does not have time to deform before it explodes.
5. What does the abbreviation SQS stand for?
– Small Quantum Systems
Mike Follows, King Edward’s School, Birmingham (UK)

How can AI systems like those developed to beat humans at games help unlock the secrets of protein…
How do physicists study very small objects (like molecules, atoms, and subatomic particles) and very large objects (such as galaxies) that cannot be…

Picture sequences provide engaging opportunities for students to explore the concepts of speed and acceleration using supplied digital images or their own smartphones.