Supporting materials
Download
Download this article as a PDF

Meet the planarian, a fascinating flatworm with incredible biological abilities and unique and surprising ways to respond to various stimuli.
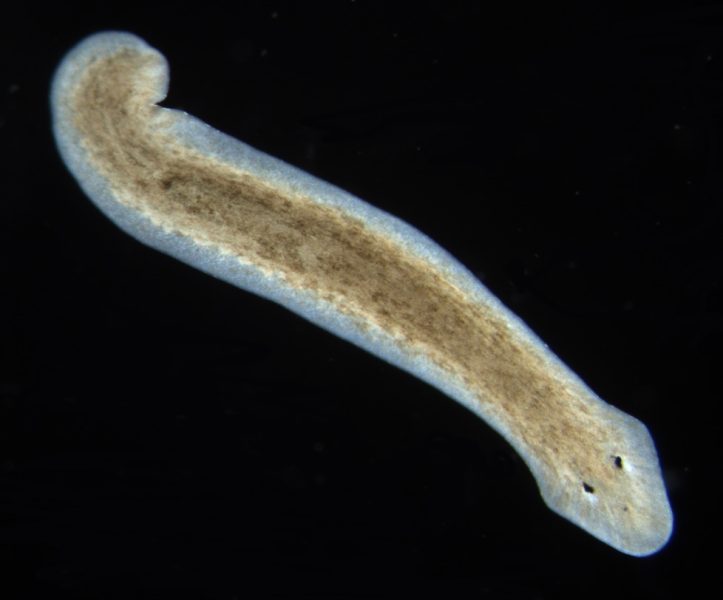
Freshwater planaria, nonparasitic invertebrate animals belonging to the phylum Platyhelminthes, the flatworms, are organisms that are well-suited to educational purposes. They are easy to maintain under laboratory conditions[1] as they require minimal resources and can survive in a simple water-based environment. Also, they are inexpensive to propagate, reasonably macroscopic (1–10 mm in length[2]), and easy to work with.

Planaria are bilaterally symmetric animals, with a complex central nervous system and two eye spots.[3]
Observing and studying nature plays a crucial role in the development of human thought. Hands-on teaching methods are often considered to be effective in the classroom, not only for explaining various biological processes, but also for fostering student curiosity.
Working with planaria allows students to gain a deeper understanding of various aspects of living organisms. By observing these remarkable creatures, students can explore organ systems, reproductive strategies, and the relationship between structure and function in living organisms. Planaria provide a hands-on learning experience that enables students to explore and comprehend these fundamental biological concepts.
We introduce a hands-on approach for students aged 14–19 to explore fundamental questions underlying animal responses to stimuli and their regeneration abilities.
Option 1. Freshwater planaria are one of about 1300 species that can be found in unpolluted streams and lakes, usually associated with the lower part of a rock or trunk. They can be easily collected by applying a light jet of water to the rock and transferring them to a container with the help of a plastic pipette, avoiding animal damage. After collection, they must be transported in containers filled with water (without air), at temperatures between 1 and 25°C. In the laboratory, at least half of the water should be replaced with chlorine-free water.
Option 2. Contact a biology department at a university. Some use planaria and are willing to provide animals for educational purposes.
The planaria care guide provides a comprehensive guide on how to prepare planarian food.
In this activity, students can investigate how these organisms react to stimuli of light, food, and touch. This allows students to gain insights into the sensory capabilities and behavioural adaptations of planaria and can contribute to a deeper understanding of their biology and ecological interactions. The activity is suitable for students aged 11–19 and will take 30–45 min to complete. The planaria infosheet can be distributed before the activity or at the end before the discussion, so students learn more about these fascinating creatures.
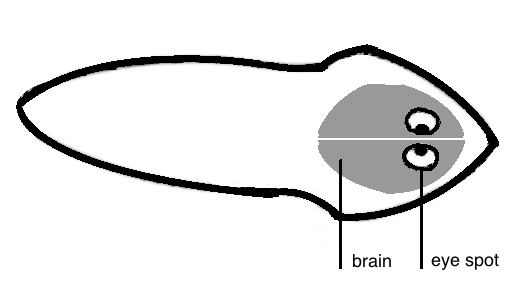
Planarians can be transferred from one container to another using plastic Pasteur pipettes with openings big enough to accommodate the worms without injuring them. If the opening of the Pasteur pipette is not big enough, the pipette tip can be cut with scissors.
Work through the worksheet and discuss the following points. Sample answers can be found on the answer sheet in the supporting material to give educators ready-to-use content for classroom conversations.
● Why do we use a new planarian for each stimulus tested?
● Compare the predictions with the experimental data. Do they match?
● Can you explain the reactions of the planarians to different stimuli?
● What is the role of the nervous system in planarian reactions?
● Why do scientists use planaria in research on the nervous system?
Finally, challenge the students to design their own planarian stimulus experiment.
Although all multicellular organisms depend on stem cells for their survival and perpetuation, planaria are capable of regenerating any missing body region[4] in a relatively short time. This regenerative ability results from the abundance of stem cells, which are the only cells with the capacity to divide and differentiate into any type of cell.[5] Planaria are particularly studied for their ability to regenerate quickly from any body fragment. They have abundant adult stem cells called neoblasts, comprising 20–30% of their cells, which can divide and differentiate into any cell type.
Regeneration is a fascinating process that replaces damaged or lost structures and requires not only new tissue production but also reorganisation of pre-existing tissues to ensure the correct size and proportion of the regenerated animal.[6] Body shape is preserved almost regardless of the type of injury, maintaining proper proportions even when the newly regenerated animal is significantly smaller than the original.[7] After amputation, there is a strong contraction of the muscles to close the cut, minimizing exposure of the internal tissues and the wound area.[8] A fragment from the previous region will continue to move, a mechanism that would allow the planarian to escape from a predator.[7] After 30 min, a thin layer of cells is transferred to the cut zone.[9] Then, stem cells increase their proliferation rate and, at the cut site, they generate new tissue without pigmentation, the blastema, where differentiation occurs, to replace the lost parts.[10] Regeneration is complete when the animal has the appropriate proportions and pigmentation is homogeneous. After two weeks, the planaria can be regularly fed again. The use of a stereoscopic magnifying glass and photographic record can aid in monitoring the evolution of regeneration.
The activity is suitable for students aged 14–19 and will take two lessons to complete.

Discuss the following questions. Sample answers can be found on the answer sheet in the supporting material.
Planaria also shrink under low-nutrient conditions. This can additionally be investigated as an extension activity, as described in the supporting material.
[1] Merryman MS, Sánchez Alvarado A, Jenkin JC (2018) Culturing planarians in the laboratory. In Rink JC (ed.) Planarian Regeneration. Methods and Protocols pp 241–258. Humana Press. ISBN: 9781493978007
[2] Dean MRP, Duncan EM (2020) Laboratory maintenance and propagation of freshwater planarians. Current Protocols in Microbiology 59: e120. doi: 10.1002/cpmc.120.
[3] Reho G, Lelièvre V, Cadiou H (2022) Planarian nociception: Lessons from a scrunching flatworm. Frontiers in Molecular Neuroscience 15: 935918. doi: 10.3389/fnmol.2022.935918
[4] Wu J-P, Li M-H (2018) The use of freshwater planarians in environmental toxicology studies: Advantages and potential. Ecotoxicology and Environmental Safety 161: 45–56. doi: 10.1016/j.ecoenv.2018.05.057
[5] Felix DA et al. (2019) It is not all about regeneration: Planarians striking power to stand starvation. Seminars in Cell & Developmental Biology 87: 169–181. doi: 10.1016/j.semcdb.2018.04.01
[6] Rink JC (2018) Stem cells, patterning and regeneration in planarians: Self-organization at the organismal scale. In Rink JC (ed.) Planarian Regeneration. Methods and Protocols pp 57–172. Humana Press. ISBN: 9781493978007
[7] Reddien PW, Sánchez Alvarado A (2004) Fundamentals of planarian regeneration. Annual Review of Cell and Developmental Biology 20: 725–757. doi: 10.1146/annurev.cellbio.20.010403.095114
[8] Levin M, Pietak AM, Bischof J (2018) Planarian regeneration as a model of anatomical homeostasis: Recent progress in biophysical and computational approaches. Seminars in Cell & Developmental Biology. 87: 125–144. doi: 10.1016/j.semcdb.2018.04.003
[9] Noreña C, Damorenea C, Brusa F (2014) Phylum Platyhelminthes. In Thorp JH, Rogers DC (eds) Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates 4th edition, Chapter 10. Academic Press. ISBN: 9780123850263
[10] Pascual-Carreras E et al. (2023) Wnt/β-catenin signalling is required for pole-specific chromatin remodeling during planarian regeneration. Nature Communications 14: 298. doi: 10.1038/s41467-023-35937-y
The article can provide valuable background reading when teaching evolution or developmental biology to students or when explaining why we must eat/provide organic substances through food to our cells or how an organism can respond to environmental cues. Also can prove how a simple invertebrate organism can help to clear some procedures which are present in complex organisms too, but we can’t use those in labs.
It is a novelty in a high school class to see how blast cells work or how an organism responds to environmental cues or how an organism can regulate its growth based on the presence or absence of food.
Alina Giantsiou Kyriacou, Biology teacher, Kykkos B’ High School, Nicosia, Cyprus
Download this article as a PDF

Future food: would you bite into a test-tube burger or a Petri dish steak? How do we make lab-grown meat, and what might it mean for health, farming, and the environment?
How do social drugs affect metabolism? How is toxicity measured? How does climate change affect water ecosystems? Promote active learning by…

Play the part: students take on the roles of different components of a synapse to act out synaptic transmission and learn about neurobiology.