Limoncello and the science of emulsions Understand article
How can you make oil and water stay mixed? A scientist’s curiosity about a lemon liqueur has revealed how to do this – with some promising industrial applications.

Jorge Royan/Wikimedia Commons, CC BY-SA 3.0
Limoncello, the fragrant Italian liqueur made from lemons, is becoming increasingly popular around the world. This sweet and citrus digestif is an iconic item of Italian food culture – but it is also a complex colloidal system made of essential oils, ethanol, sucrose and water.
As an Italian chemist working at the Institut Laue-Langevin (ILL)w1, I was curious to find out what ILL’s advanced technology might reveal about this complex system. So, earlier this year, my colleagues and I applied for beamline time to conduct a small study, and it turns out that – as well as being delicious – limoncello has some rather special scientific characteristics.
What is limoncello?
In the traditional limoncello recipe, the citrus zest (obtained by scraping the outer part of the lemon peel) is macerated in alcohol (ethanol) for several weeks. The zest contains most of the essential oils in lemons, producing the characteristic taste and colour of the liqueur. The ethanol and lemon extract is then mixed with a sugar syrup. Limoncello typically contains approximately 30% alcohol and around 20% sucrose (sugar) by volume, but as limoncello is often home made, the preparation method and final composition vary from family to family.
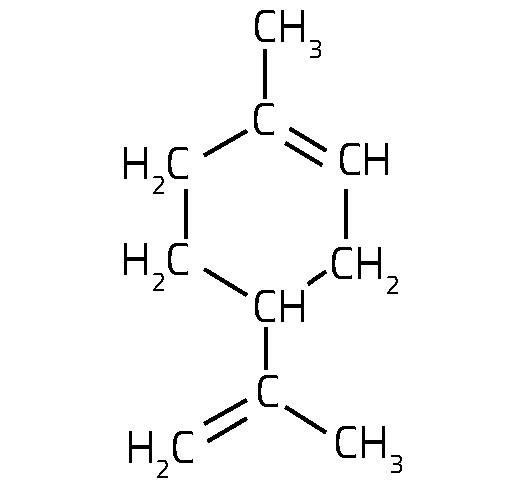
limonene
Nicola Graf
The essential oils so important to the flavour of limoncello are found in small pockets in the peel of citrus fruits, which burst and deliver the typical strong scent we notice when peeling such fruit. These essential oils have a very complex composition: more than 60 different molecules have been identified, with a class of organic molecules called monoterpenes being the main components. In lemons, the most abundant compound is limonene (figure 1).
Limoncello is made by mixing two solutions: the ethanolic extract containing the oils, and the aqueous sucrose solution. Each of these starting solutions is completely transparent; limoncello itself, however, is ‘turbid’, with a cloudy, opaque appearance. Turbid systems pervade everyday life: other examples include ice crystals in clouds, fat droplets in milk, and algae in a pond. These different systems all contain particles or droplets at the scale of hundreds of nanometres, which is comparable to the wavelength of visible light. It is these ‘heterogeneities’ – tiny amounts of solid or liquid suspended in a fluid medium – that give these systems their turbid appearance.
The ‘ouzo effect’
So where does the turbidity of limoncello come from? Water and ethanol are fully miscible (soluble in each other), as are limonene and ethanol – but limonene and water are barely miscible. In limoncello, this combination of three liquids spontaneously produces an ‘emulsion’: a suspension of tiny droplets of one liquid in another. However, this only happens at some specific ranges of composition (see text box).
This phenomenon of spontaneous emulsion formation is called the ‘ouzo effect’, after the famous Mediterranean drink called ouzo that immediately becomes turbid when mixed with water, forming an emulsion. Indeed, ouzo is, from a scientific perspective, quite similar to limoncello, as it is made from water, ethanol and the flavour component anethole, which – like limonene – is highly soluble in ethanol but only slightly soluble in water.

canbilgic/Shutterstock.com
In contrast to these ouzo systems, typical emulsions require a very high energetic input – such as the shaking and stirring required to make the emulsion we call mayonnaise. Another very important difference between ouzo systems and classical emulsions is the absence of any stabilising agents. For example, mayonnaise is prepared by emulsifying a vegetable oil with the water contained in egg yolk. The process is long and tedious, and it requires a substantial amount of energy – provided by vigorous shaking and stirring – to make the two liquids mix to form the emulsion. Lecithin and proteins contained in the egg yolk are also needed to stabilise the emulsion.
So why are ouzo systems important outside the kitchen? Some important industrial processes take place in emulsions – for example, polymerisation, where small molecules (monomers) combine to form large macromolecules, or polymers. Here, emulsions are often created to bring the reagents into close proximity so the reaction can proceed quickly. If such emulsions form spontaneously (as in limoncello), requiring very little energy input, if any, this obviously makes the process more efficient and sustainable. In addition, the polymer product needs to be recovered from the reaction medium at the end of the reaction, which is often the most challenging step of the entire process. However, if the system contains no stabilisers, the extraction of the polymer and catalysts is much simpler, as the components can easily separate once the emulsion-forming composition no longer exists. Another widely used application of emulsions is in pesticides, to enable these water-insoluble products to be diluted and spread onto fields. Using an ouzo-type emulsion would avoid also spreading unnecessary surfactants, which are often harmful to the environment.
Limoncello at the micro level
used for the limoncello study
A Chezière/ILL
As mentioned, the way limoncello scatters light is quite revealing about the liquid’s structure at the microscopic level. Using radiation of shorter wavelength, X-rays or neutron beams allow us to look in more detail at structures and interactions within this liquid, and at an even smaller scale.
We hoped to use the neutron scattering facilities at ILL to see what they could tell us about limoncello – and, luckily, we were allocated time on the small-angle neutron scattering (SANS) beamline. The aim of our research was to find out where the extraordinary stability of limoncello comes from. With this aim, we studied the liqueur under different conditions: when water is added to the ethanolic extract; at different temperatures; and at different sucrose concentrations (Chiappisi & Grillo, 2018). Neutrons are sensitive to the isotopic composition of the system, and they interact very differently with the two stable isotopes of hydrogen: protium, 1H (normal hydrogen), and the much rarer deuterium, 2H. In the research, the essential oil was extracted from a lemon bought in the local market (thus containing mostly protium nuclei), while the ethanol and water were highly enriched with deuterium nuclei, as a contrast.
The analysis revealed that, in limoncello, the size of the oil-rich domains is always around 100 nanometres in diameter, regardless of water content, sugar content or temperature. These results are surprising: the typical size of the oil-rich domains in ouzo systems is normally much larger, at several hundreds or even thousands of nanometres in size (Grillo, 2003). In addition, their size is usually very sensitive to the composition or the temperature of the system – unlike in limoncello.
This makes limoncello a very interesting liquid, scientifically. The small size of the oil droplets seems to provide its exceptional stability in relation to changes of temperature and composition, and also over time. In fact, limoncello can be kept in the bottle for years: not bad for a metastable system! In contrast, drinks like pastis or ouzo tend to phase separate within a few hours of preparation (which is why a pastis is always diluted with water in the glass, just before consumption).
So while we don’t yet fully understand why limoncello behaves so differently to other ouzo-type drinks, we now have a better understanding of the science of self-emulsifying systems – and how to design them for use in future products and processes.
Phase diagrams and the stability of limoncello
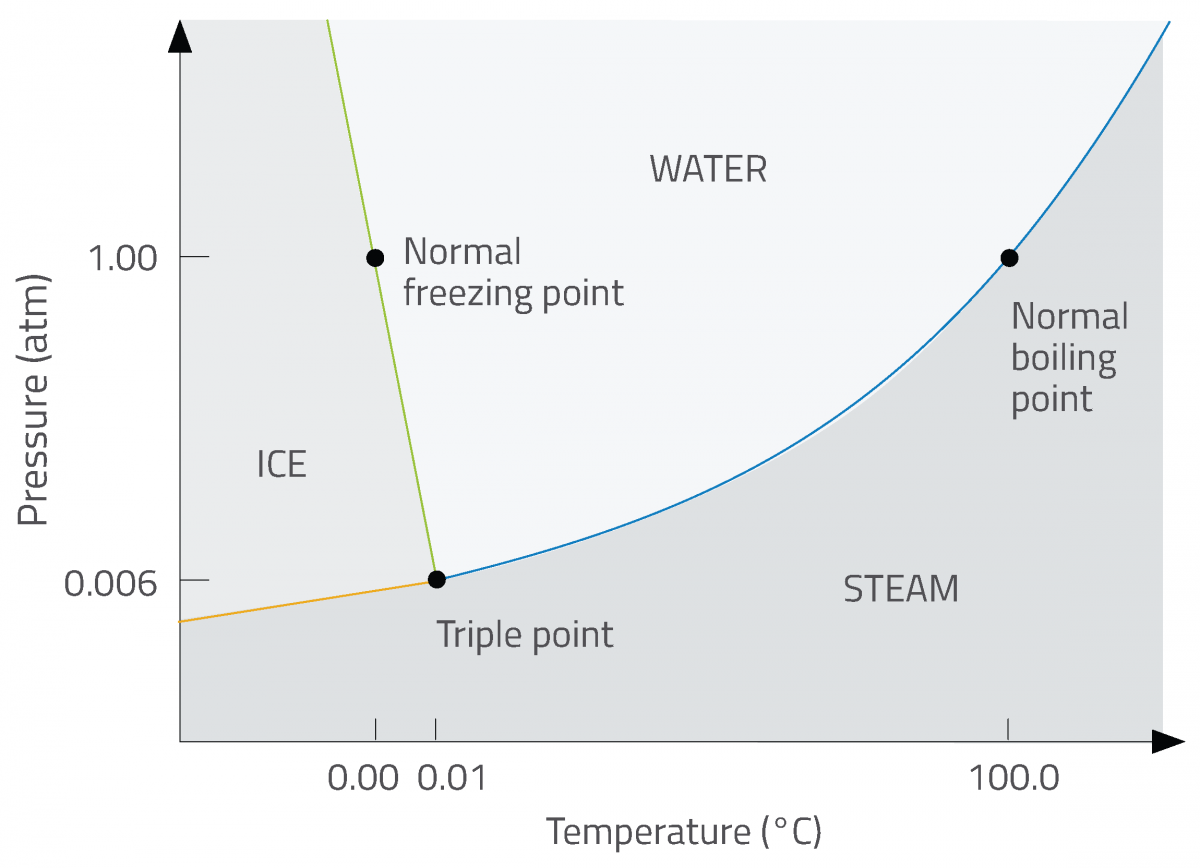
Phase diagrams are a convenient way of representing the changing physical states of systems of two or more components under different conditions. A common type of phase diagram shows how a single substance (water, for example) will change state between solid, liquid and gas at different combinations of temperature and pressure (figure 2).
Nicola Graf/Leonardo Chiappisi
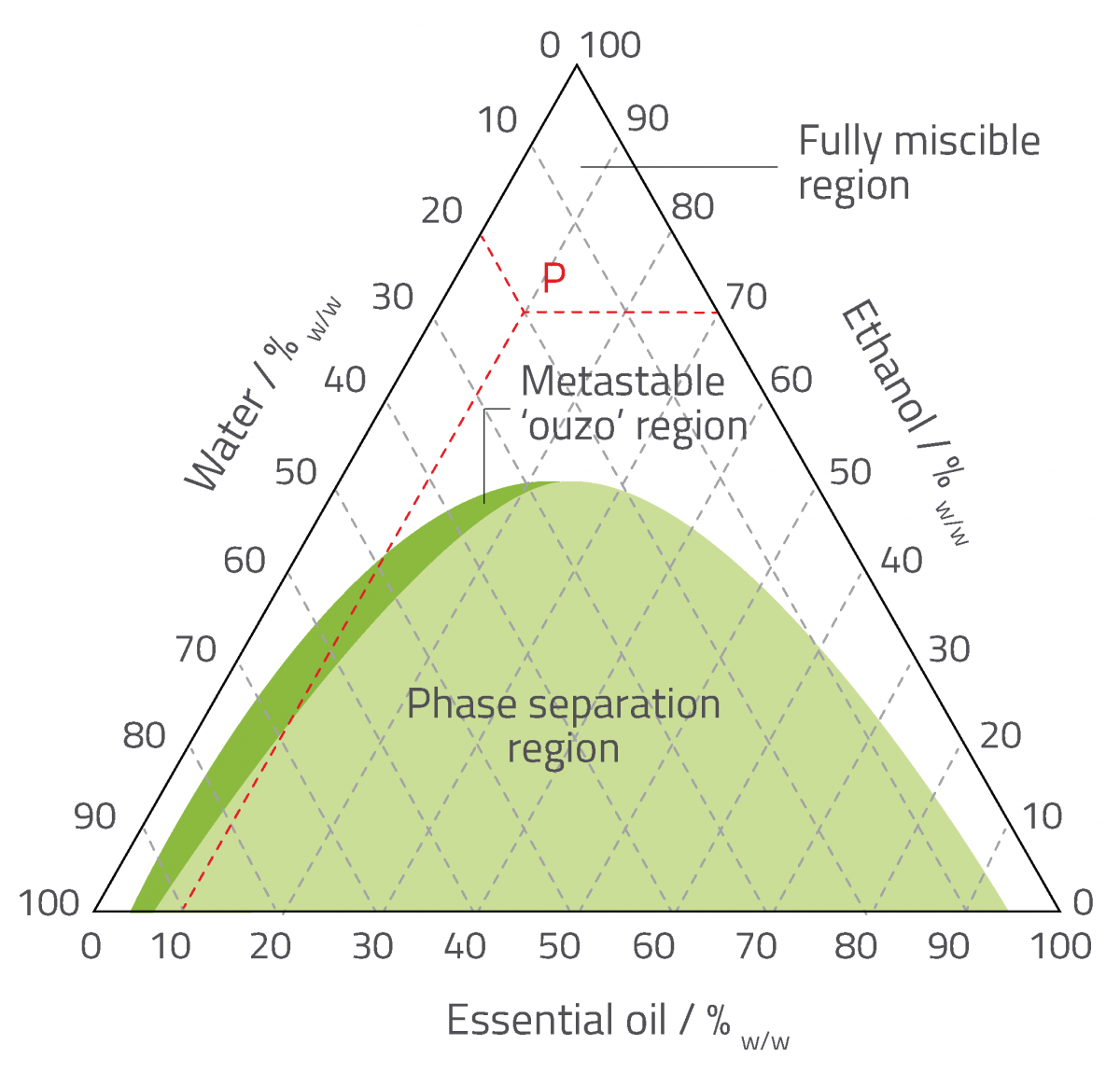
In systems like limoncello, which is itself made up of three components (water, ethanol and essential oil), phase diagrams allow us to represent the possible compositions within the system, and the physical characteristics (such as solubility and stability) associated with each composition. A typical three-fold phase diagram is shown in figure 3. Here, each of the pure components is represented by a vertex of the main triangle, where the adjoining scales read 100% and 0% for two different components.
representing an ouzo system
Nicola Graf/Leonardo Chiappisi
As the example illustrates, the composition of a point within the phase diagram can be read by drawing three lines starting from the point and ending at each axis (note the triangular grid used to draw the lines). In this case, a sample represented by the red point P will have a composition of 20% water, 70% ethanol and 10% essential oil (w/w).
From this phase diagram, we can see that water and ethanol are fully miscible, as are ethanol and the essential oil. However, the solubility of the essential oil in water is only 5% w/w, and the solubility of water in the essential oil is less than 10%. The diagram also shows an area in which the components separate into at least two phases and don’t mix (the phase separation region). The small ‘metastable ouzo region’ is where the composition enables spontaneous emulsion formation – as found in ouzo systems. Phase separation will occur eventually in this region, but the timescale can be very long because the metastable state requires energy to overcome.
References
- Chiappisi L, Grillo I (2018) Looking into Limoncello: the structure of the Italian liquor revealed by small-angle neutron scattering. ACS Omega 3: 15407-15415. doi: 10.1021/acsomega.8b01858
- Grillo I (2003) Small-angle neutron scattering study of a world-wide known emulsion: Le Pastis. Colloids and Surfaces A: Physicochemical and Engineering Aspects 225: 153-160. doi: 10.1016/S0927-7757(03)00331-5
Web References
- w1 – Based in Grenoble, France, ILL is an international research centre at the leading edge of neutron science and technology.
Resources
- The study of the microscopic structure of limoncello was carried out using the SANS instrument D11 at the ILL. Learn how this instrument works on the ILL website.
- Find out more about how neutron scattering works from an article from ILL about a study of how some bacteria can live in salty sea environments. See:
- Zaccai G (2018) Titanic and the iron-eating bacteria. Science in School 43: 8-11.
- Read about how SANS was used to develop a new, recoverable surfactant at the ILL. See:
- Eastoe J et al. (2012) Magnetic science: developing a new surfactant. Science in School 25: 22-27.
Institutions
Review
Most students will recognise that oil and water do not mix, and they may have heard the word ‘emulsion’ when helping a family member paint a room – but despite these real-world examples, very few will have questioned the chemistry behind such experiences. By encouraging students to question what is happening on a macro level and inspiring them with the chemistry occurring on a micro level, this article provides an accessible gateway to some key concepts.
In addition, there is an opportunity to interpret a three-fold phase diagram, which allows students to use their mathematical skills to derive conclusions about physical characteristics, demonstrating that such skills are essential for scientific research.
Comprehension questions that could be used in class include:
- The limonene molecule has two possible enantiomers. Identify the chiral carbon atom.
- What volume of ethanol would you expect 5 litres of limoncello to contain?
- Turbid systems contain particles at a scale comparable to the wavelength of visible light. What is the wavelength range of visible light?
- Why could the science of limoncello prove useful for designing self-emulsifying systems?
- The phase diagram contains a ‘metastable ouzo region’. What is meant by the term ‘metastable’?
Caroline Evans, Head of Chemistry, Wellington College, UK





