The mathematician who became a biologist Inspire article
Theodore Alexandrov is taking what he learned from working on the economy and applying it to the chemicals on our skin.
You might not think that your body’s biology has much in common with the workings of your credit card or the global banking system – and neither, at first, did Theodore Alexandrov. But Theodore is now using mathematical algorithms for understanding the economy to analyse information about the countless molecules produced by our cells.
Theodore and his team are developing a new technology that maps where these molecules are in relation to each other in three-dimensional (3D) space. The work is leading to a new spatial understanding of biological processes, such as the metabolism of our cells and the interactions between microbes in the environment, as well as offering insights into how they can go wrong. “If we want to truly understand how all these processes work, then we need to see where all these molecules are,” says Theodore.
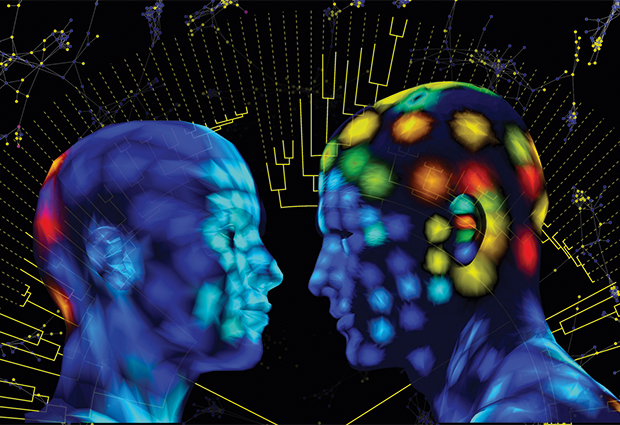
Image courtesy of Theodore Alexandrov
The field in which Theodore’s team works is known as metabolomics: the study of the biochemical fingerprints produced by the reactions in our cells. A person’s metabolome is hugely complex and dynamic: doing something as innocuous as drinking a cup of coffee or eating a sandwich, for example, dramatically alters the mix of substances produced by our cells – within seconds. Different kinds of cells and tissues have different metabolomes, which can change as a result of disease or a changing environment. Researchers are keen to understand these variations to gain new insights into both normal and abnormal processes in our bodies.
Molecular maps
To identify molecules in a sample, researchers usually turn to a method known as mass spectrometry, or mass spec for short. This technique involves ionising the molecules and then using electric and magnetic fields to ‘weigh’ each one. The machine produces patterns known as spectra, which researchers can then interpret. However, some molecules come in a range of slightly differing forms, so the spectra of samples containing many molecules can be extremely difficult to decipher.
New developments in the field are providing even more information for scientists to contend with. For example, imaging mass spec not only identifies molecules but also determines their location in space. Scientists can place a thin section of a tumour or cell culture on a microscope slide and use a laser to systematically vaporise the molecules within it, point by point. They can then cross-reference the molecules they find to the points on the slide from which they originate. Putting this location information together with the mass spec data requires powerful software – software that Theodore was in an ideal position to develop thanks to his background.
Maths to mass spec
Image courtesy of EMBL
Photolab / Marietta Schupp
Having completed a PhD in mathematics and statistics in his home town of St Petersburg in Russia, Theodore embarked on a postdoctoral research project in Bremen, Germany, predicting bank transactions over time for a credit-card company. There, a colleague pointed out that Theodore’s expertise in developing algorithms to track how things change over time could also be applied to the rapidly growing field of mass spectrometry. “For me this is the perfect field,” he says. “We’re working with gigabytes, even terabytes of data, and my background in mathematics really helps.”
In 2012, together with Pieter Dorrestein from University of California, San Diego, USA, Theodore came up with the idea of creating maps of metabolites on the human skin. This rapidly grew to involve more international colleagues and to map both metabolites and microbes on the skin. By combining mass spec and imaging information, the team was able to create a 3D map of the molecules clinging to the skin of two volunteers. What’s more, they also correlated this map with information on the distribution of different microbial species. “What’s unique about our approach is that we always try to bring spatial information into the analysis,” Theodore says.
Molecules and microbes
Thanks to this spatial analysis, researchers now have a better picture of the molecular environment of our skin’s surface. They also have a starting point to understand more about how this environment might affect our skin’s relationship with its resident microbes. The interconnections between our cells and the trillions of microbes that inhabit our bodies are currently of intense research interest, as evidence mounts that these microbes have a profound influence on our health (see Viosca, 2015, to read about the microbial colonies in our mouths).
Moving mass spec into the third dimension promises to revolutionise many areas of research. “It is now being used in clinics, biomedical research and pharmacy,” explains Theodore. One key example is cancer research. Individual tumours contain cells that differ from each other. Understanding these differences is therefore very important if we are to learn more about how the disease develops and progresses. “One cell can change everything,” says Theodore. “Now, we can see molecular differences between the cells.”
Community conquests
Since joining the European Molecular Biology Laboratory (EMBL)w1 in November 2014, Theodore and his team have been working on two technological projects. The first is a continuation of his collaboration with Dorrestein’s group, and involves developing bioinformatics tools to map the spatial distribution of molecules in any environment. The second project involves developing new algorithms and hardware infrastructure so that users can identify more of the molecules in their samples. At the moment, the fact that one molecule can generate hundreds of different signals in a mass spec machine means that scientists can only interpret a small fraction of their spectra. “I’m pretty sure we’re just scraping the surface: we’re not getting the full molecular snapshots,” says Theodore. This work forms the basis of a European project that Theodore is co-ordinating, bringing together eight partners from academia and industry. “They are all interested in really getting this working as a community effort,” he explains.
But even as his team grapples with the challenges of moving mass spec into the third dimension, Theodore already has his sights set on the fourth. Being able to monitor metabolomes in time as well as space will let scientists track biological processes as they happen. “It’s still very preliminary, and we would like to improve the resolution to really understand this complexity,” he says. “But we can already see how metabolomes change over time.”
More about EMBL

The European Molecular Biology Laboratory (EMBL)w1 is one of the world’s top research institutions, dedicated to basic research in the life sciences. EMBL is international, innovative and interdisciplinary. Its employees from 60 nations have backgrounds including biology, physics, chemistry and computer science, and collaborate on research that covers the full spectrum of molecular biology.
EMBL is a member of EIROforumw2, the publisher of Science in School.
References
- Viosca J (2015) A safari in your mouth’s microbial jungle. Science in School 34: 16-18
Web References
- w1 – Get more information about EMBL.
- w2 – EIROforum is a collaboration between eight of Europe’s largest inter-governmental scientific research organisations, which combine their resources, facilities and expertise to support European science in reaching its full potential. As part of its education and outreach activities, EIROforum publishes Science in School.





