LeSa21: primary-school science activities Teach article
Teaching science in primary school can be challenging. Astrid Kaiser and Marlene Rau describe a rich source of online materials in three languages – and highlight some activities about oil and water.

Storch; image source:
Wikimedia Commons
Launched in 2002 by Astrid Kaiser from the University of Oldenburg, Germany, the LeSa21 websitew1 offers a range of experiments, resources and background information for primary-school science and humanities lessons. Materials for both teachers and their pupils are available on a wide range of topics (currently 93), including ‘The eye’, ‘bicycles’, ‘spring’, ‘health and disease’, ‘girls and boys’, ‘planets, the Moon and the stars’, ‘electricity’, ‘water’ and many others. These materials are developed mainly by university students who are training to be primary-school teachers.
Available in English, German and Spanish, the ‘Learning’ section is suitable for both children and teachers, and includes experiments, stories, poems, pictures, book recommendations and links to relevant websites for each of the topics.
The pages for teachers (in German only) contain information, didactical hints, literature and web links to background information and further resources on each of the topics. Pupils at the six schools involved in the project contribute to further German-language sections. There, they can share their own experiments and ideas on the topics, and discuss a selection of the topics in an online forum.
Below are some activities from the LeSa21 project, which could form the basis of a teaching unit on oil and water, including surface tension and the removal of oil spills, suitable for older primary-school children. The individual experiments and further materials on the topics can be found on the LeSa21 websitew1.
Soap has magic powers – surface tension
This experiment introduces the notion of surface tension.

Image courtesy of LeSa21
Materials
- Two bowls of water
- Ground pepper
- A pin
- A spoon (the size is not important)
- Liquid soap or washing-up liquid
Procedure
- Half fill both bowls with water.
- Sprinkle a spoonful of pepper onto the water in the first bowl. Take a close look while you are doing this.
- Let the pin float in the second bowl of water.
- Add a drop of soap to each bowl.
- What happens? Draw what you observe.
What happens?
Initially, both the pepper and pin float. One reason is that they are both very light; the other is that water has a kind of thin, invisible ‘skin’ on its surface. Light things do not break this skin, but are supported by it – some animals, e.g. water striders, can even walk on it (see image above). This thin skin is caused by surface tension.
When you added the soap drop, the pepper probably moved away from it and slowly sank. The pin would have sunk immediately.
Why is that? The soap breaks this thin skin of the water – you could also say that soap reduces the surface tension. As the skin disappears, there is nothing left to hold the pepper and the pin on the surface of the water, so they sink. Although this is how it may look, pepper and soap do not repel each other – the tension of the rest of the water (where the soap has not yet reached) pulls the floating pepper away from the soap.
Soap has magic powers – mixing oil and water

Image courtesy of LeSa21
Materials
- Two glasses
- Water
- Food colouring or ink
- A spoon (the size is not important)
- Oil
- Liquid soap or washing-up liquid
Procedure
- Half fill the glasses with water.
- Add some food colouring or ink to one glass, and mix it with a spoon. Leave it for a while until the water is still again.
- Add a spoonful of oil to each glass and observe what happens.
- Add 1-2 drops of soap to each glass, and mix it with a spoon.
- What do you think will happen? Observe carefully and draw what you can see.
What happens?
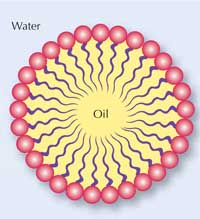
Oil and water usually do not mix well. The reason is that water molecules can form hydrogen bonds with each other and with hydrophilic (‘water-loving’) molecules, but not with oil-like hydrophobic (‘afraid of water’) molecules. In addition, oil is lighter than water; it has a lower density, so if you try to mix them, the oil layer stays on top of the water. If you add some soap and stir it for a while, the oil and water can be mixed together. If you leave it for a while, the oil and water will separate again.
Why is that? Soap contains special ingredients called surfactants, which reduce the surface tension of water and allow water and oil to mix. This makes soap a wonderful cleaning agent: not only water-soluble dirt but also oily dirt can be washed away into water.
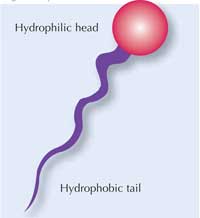
Surfactant molecules have two different ends, as shown in the diagram (right). One end (the head) is hydrophilic; the other end (the tail) is hydrophobic. Soap contains many surfactant molecules, and each of them binds water at one end and oil at the other end, which is how they help water and oil to mix, and also bind to oily dirt.
droplet
Images courtesy of Nicola Graf
After a while, the oil and water separate again, because the bonds between water molecules are more stable than those between the surfactant molecules and the oil or the water.
The oil spill

the blaze on the oil rig
Deepwater Horizon on 21
April 2010
Image courtesy of US Coast
Guard; image source:
Wikimedia Commons
In April 2010, an explosion on the oil rig Deepwater Horizon in the Gulf of Mexico led to a catastrophic oil spill. As we go to press, an estimated 5000-100 000 standard barrels of oil per day have been leaking out for several months, the spill eventually reaching the southern coasts of the USA. Attempts to remove the oil included burning it and using chemical and mechanical binding agents.
Similar accidents have happened in Europe, too: in Autumn 2002, the oil tanker Prestige was involved in an accident off the Spanish coast and sank. Up to 63 000 tonnes of oil were spilt into the sea, and hundreds of kilometres of the coastline of northern Spain and France were polluted with oil. Many animals died: more than 18 000 oil-contaminated birds were counted. Many volunteers helped to clean the beaches, and special ships tried to stop the growing oil slicks.
Let’s find out what oil does to bird feathers and how we can remove the oil from the water.
Materials
- Several big glasses or china bowls
- Water
- Vegetable oil
- Feathers
- A spoon
- A paper coffee filter
- A straw
- Wooden sticks
- Fine sawdust

Step 1 - Wood shavings
- Cat litter
- Sand
- Oil binding / absorbing powder (used for oil spills, can be bought online or in well-stocked paint shops or chemists)
Procedure
- Pour some oil into a bowl and add some feathers.

See how they stick together and imagine what effect an oil spill would have on birds and other animals, once the oil reaches the shores.
How might oil spills be cleaned up in open water, to prevent them from reaching the shores?
We will test various methods of removing the oil from the water. Either ask the children to get into groups, with each group testing a different method, or let the whole class watch as you try out all methods together.
- Take a new bowl, fill it with water, add some oil and stir. What happens?
- Skim off the oil with a spoon.

Step 3 - Skim off the oil with a coffee filter.
- Try to push the oil together with sticks.
- Mix the oil with wood shavings. Now try to push the oil-soaked wood shavings together.
- Does it work better with cat litter? If so, why?
- Bind the oil with sawdust (it will float due to the oil’s hydrophobicity and lower density) and remove it from the water using a spoon or paper coffee filter.
- Try to weigh down the oil with sand to make it sink.
- Use oil binding / absorbing powder to bind the oil and remove it using a spoon.

Images courtesy of LeSa21
Compare and discuss the results of the experiments. The children should realise that the best results are achieved with a combination of (mechanical and chemical) methods. They may also consider the factors affecting the efficiency of each method, e.g. the volume of removal agent used. Finally, they should notice that there will always be some oil left in the water at the end; in real-life situations, we have to rely on the ecosystem to deal with this remainder over time.
Which other methods of cleaning the water can you imagine? Let the children come up with some ideas for real-life ‘oil spill removal machines’. Ideas might include a ring of cat litter to stop the oil from floating away, plus mechanical diggers to remove the floating oil film.
Comprehension questions
- What happens if you pour oil into water?
Answer: oil and water don’t mix – the oil floats, forming iridescent streaks and / or grease drops. - How can you separate the oil from water?
Answer: using a combination of the above methods, but it is difficult. - Is the water clean again after your experiment?
Answer: no, some oil will always remain. - What is left in the water?
Answer: oil, some of which is clumped together with cleaning agents.
The origins of Lesa21: boxes of experiments
The origins of Lesa21 go back to the RÖSA projectw2, which is still continuing at the University of Oldenburg. Since 1994, images, stories, experiments and background information for primary-school science and humanities lessons (Sachunterricht) have been sorted by topics and collected in boxes that local teachers can borrow.
The experimental equipment in the boxes is mostly recycled material found in standard households or industry, such as broken mirrors, pipettes from empty medicine bottles, boxes of old buttons, stones and used guitar strings. The objects not only provide an inexpensive source of experimental equipment, but also demonstrate to the children that materials can be used over and over again in different and creative ways. Individual boxes have been created on 70 different themes, many of them the same as the current LeSa21 topics. The ideas and materials are tested in local schools by university students and trainee teachers.
Several satellite Lernwerkstätten offer similar collections of boxes, mainly in schools across northern Germany, but also at the Pädagogisches Beratungszentrumw3 (education centre) in Brixen, northern Italy. In addition, a book has been published for Japanese teachers, who are now also working with the box system (Kaiser et al., 1999).
Acknowledgements
The two experiments ‘Soap has magic powers’ are part of the book Chemie in der Grundschule (Chemistry in Primary School; Kaiser & Mannel, 2004). Published in German, it contains many other experiments on different topics.
Parts of ‘The oil spill’ experiment are from Kaiser (2009).
References
- Kaiser A et al. (1999) Topic – bezu Sogo gakushu. Doitzu no Kyoiku tono Sissen taiwa – Nisiu itteheikino Gakkou wo hiraku. Kyoto, Japan: Kitaoji-Shobo. ISBN: 4762821578
- Kaiser A, Mannel S (2004) Chemie in der Grundschule. Baltmannsweiler, Germany: Schneider Verlag Hohengehren. ISBN: 9783896767660
- Kaiser A (2009) Praxisbuch handelnder Sachunterricht Band 1 3rd edition. Baltmannsweiler, Germany: Schneider Verlag Hohengehren. ISBN: 3834000167
Web References
- w1 – For more information about LeSa21, see: www.lesa21.de
To reach the English and Spanish versions of the ‘Learning’ section, click on ‘Lernen / Learning / aprendizaje’ in the top menu, then choose your language flag on the left-hand side. - w2 – To learn more about the RÖSA project, see: www.roesa.uni-oldenburg.de
- w3 – Find out more about the Pädagogisches Beratungszentrum in Brixen, Italy, here: www.schule.suedtirol.it/pbz/brixen
Resources
- The Australian Maritime Safety Authority has developed a series of very good educational resources for primary- and secondary-school children about oil spills, including several games, animations and the mathematics of oil spills. See the website (www.amsa.gov.au) or use the direct link: http://tinyurl.com/375ny3e
- You might also want to browse the ‘Bridge’ collection of free marine education resources from the Virginia Institute of Marine Science, USA, searching for ‘oil spill’: www2.vims.edu/bridge
- The US National Wildlife Federation offers a special section on the 2010 oil spill in the Gulf of Mexico, including background information and teaching activities. See the website (www.nwf.org; search for ‘oil spill school’) or use the direct link: http://tinyurl.com/36sqg45
- The US National Oceanic and Atmospheric Administration has developed a comprehensive teaching module on how an ecosystem recovers from a major oil spill, based on the Exxon Valdez disaster 1989 in Alaska’s Prince William Sound (‘Prince William’s oily mess’). See the website (http://oceanservice.noaa.gov) or use the direct link: http://tinyurl.com/ofvaxk
- The US education organisation The League offers a four-lesson plan on oil, water and wildlife: http://learningtogive.org/lessons/unit377
- For a broader consideration of hydrocarbon fuels, see:
- van Dijk M (2009) Hydrocarbons: a fossil but not (yet) extinct. Science in School 12: 62-69. www.scienceinschool.org/2009/issue12/energy
Review
The Lesa21 website contains a very handy and convenient alphabetical list of topics and associated activities for primary school. A good number of these are not restricted to the science classroom, which makes integration between different subjects easier. The topics can therefore also be used as inspiration for interdisciplinary secondary-school projects.
The teaching activities detailed in this article may be used as a model of how real-life situations, in this case oil spills, can be used as a starting point for learning science. The article should be of particular interest to teachers in coastal areas, which are the first to suffer the consequences of such disasters.
The experiments are interesting and may be used on their own or in conjunction with other activities such as comprehension exercises and discussions. Questions to ask could include “What are the main challenges during an oil clean-up operation?” (e.g. weather conditions), and “What can we do on an individual level to minimise the possibility of such disasters?” (e.g. reduce oil consumption). One can also look at the implications for the individual, such as how to dispose of cooking oil, or how to clean something covered in oil.
Paul Xuereb, Malta





