The importance of failure: interview with Paul Nurse Inspire article
Paul Nurse’s failed experiment inspired a Nobel-prizewinning career.

Image courtesy of Rosie Hallam
When Paul Nurse was a student in the 1960s, scientists knew that cells divide and make copies of themselves. Yet key questions remained a mystery: what drives cells to divide? What controls these divisions? How is the copying of DNA initiated? Gripped by these puzzles, Nurse devoted a large part of his career to identifying crucial mechanisms underlying the cell division process – work that ultimately won him a Nobel Prize. Yet things could have turned out very differently.
“I had very good grades in school and was offered a place at every university I applied for”, says Nurse, who now heads the Francis Crick Institute in London, UK. “However, the offers were conditional on me passing a very elementary French exam, and I failed it six times – it’s not like I wasn’t trying, but I am completely incompetent at languages.”
Against the odds
Still struggling with his French, Nurse left school and spent time working as a technician in a laboratory run by a local Guinness brewery. Each week he quickly completed his work, leaving plenty of time for research projects, which he loved. But despite repeated attempts, he just could not pass the French exam. It took a chance encounter with genetics professor John Jinks to ignite Nurse’s scientific career. Jinks recognised his potential and arranged for him to enrol as a biology student at Birmingham University, UK. “There was a sting in the tail because the university insisted I study French in my first year!” Nurse recalls.
More hurdles followed. “I was initially interested in ecology, but a field trip collecting specimens in freezing waters taught me I was better suited to the warmer environment of the lab”, Nurse says. It was there, under the guidance of an eccentric zoology lecturer, Jack Cohen, that he undertook a project measuring the respiration rate of dividing fish eggs.

Shutterstock.com
“Cell division is the basis of all growth and development – I was immediately fascinated by it”, Nurse recalls. Over the course of the following months, he carefully collected eggs from the university aquarium, placing samples in a sealed chamber. He then measured ambient oxygen levels, painstakingly observing the effects of different inhibitors. “I soon saw that the respiration rate oscillated every fifteen minutes or so, which is also roughly the time needed for the fish eggs to divide”, he says. “Strangely this pattern persisted no matter what I did to the system – it seemed incredibly robust.”
Yet a week before Nurse was due to hand in the work, a seemingly routine control test left him stunned. “I ran the experiment with no eggs in the chamber and I measured the same, perfect, oscillation”, he says. “I repeated the experiment again and again, convinced there must be a mistake. But I eventually realised that rather than measuring the respiration rate of the eggs, all the time I had been monitoring the effects of a thermostat in my apparatus. It was a complete failure from beginning to end.”
With his grades at stake and just one week to go before presenting the study, Nurse faced a big problem. “The only thing that I could think of to salvage my degree was a piece of theatre”, he recalls. “In my presentation I re-lived the whole study, from its exciting beginnings to its disastrous ending – and somehow the audience was impressed. One key message was: do controls early on in your study as soon as it becomes interesting!”
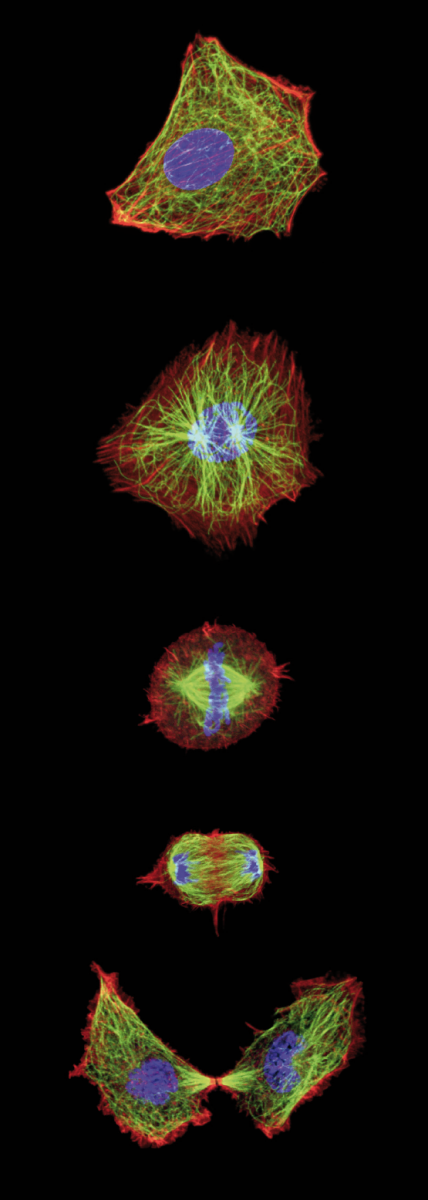
cell cycle, the chromosomes
separate as daughter cells
are formed.
Image courtesy of Jan Ellenberg
Keep going
“At my low points, I contemplated alternative careers,” he says, “but I am very much an experimentalist at heart and I have been lucky over the course of my career to have had very supportive colleagues.” Ultimately undeterred, Nurse successfully completed his degree and PhD. As a postdoc, he saw the cell cycle as a way to learn more about what fascinated him most: the nature of life. “The cell is the simplest thing that demonstrates life”, he says. “Key to understanding that is knowing how information is managed in the cell to generate order in space and time.”
Inspired by studies showing how genetics could be used to study the budding yeast cycle, Nurse returned to a research subject that he had first encountered when working in the Guinness laboratory: brewer’s yeast. “I wanted a model organism that would be simple and effective”, he recalls. He led work that treated yeast in a way that induced mutations randomly in genes throughout the yeast genome.
Nurse figured that the key to identifying genes controlling cell division in yeast would come from studying cells that divide particularly slowly (creating bigger cells) or particularly quickly (creating smaller cells). The second category was discovered by chance when Nurse observed some unusually small cells that divided more rapidly before they could grow. He identified a mutation in a gene called cdc2 that appeared to play a role in initiating key stages of the cell division cycle. “Sometimes nature provides the best leads”, he says.
After discovering that a cdc2-like gene was also in another type of yeast, Nurse wondered if the gene might exist in all organisms – a question he began to tackle at the UK’s Imperial Cancer Research Fund laboratories in 1984. “There were a few eyebrows raised as to what exactly a yeast researcher was doing at a cancer research centre”, he says. His team took a human gene library and added it to yeast lacking the cdc2 gene. Incredibly, after one of the human genes was added to the yeast, the cells divided as normal. This observation enabled Nurse to draw the astounding conclusion that a fundamental engine driving the cell cycle was the same in all species, a mechanism that had traversed 1–1.5 billion years of evolution.
The work led to the discovery, with friend and colleague Tim Hunt, of cellular messenger molecules called cyclin-dependent protein kinases – cellular messengers that pass on signals – and other insights into the nature of the cell cycle, all crucial for understanding health and disease. In 2001, Nurse, Hunt and Leland H Hartwell were awarded the Nobel Prize in Physiology or Medicine for their discoveries of key regulators of the cell cyclew1.
“It is important to know the real stories behind science and the failures and successes that are part and parcel of our work to inspire the next generation of scientists”, Nurse adds. “There is still a lot we don’t know about how cells organise in space and time, but I think we will make real progress in the coming half-century because of the methodologies that we have developed in the past five decades. And, of course – as I learned from my fruitless experiments on fish egg respiration – from the countless failures we have made along the way.”
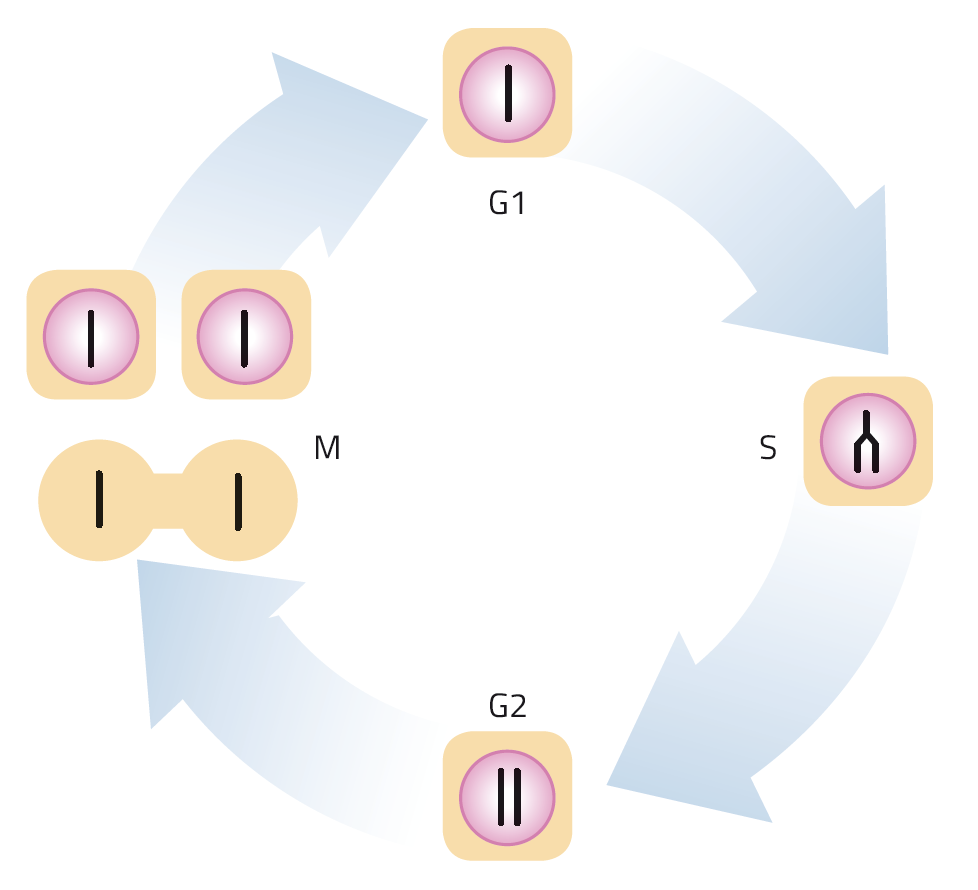
Image courtesy of Nicola Graf
Acknowledgement
The original version of this article was published in EMBLetc, the magazine of the European Molecular Biology Laboratory (EMBL)w2.
Web References
- w1 – In 2001, the Nobel Prize in Physiology or Medicine was awarded jointly to Leland H Hartwell, Tim Hunt and Sir Paul M Nurse. Read more about Sir Paul Nurse on the Nobel Prize website.
- w2 – EMBL is one of the world’s top research institutions, dedicated to basic research in the life sciences. EMBL is a member of EIROforumw3, the publisher of Science in School.
- w3 – EIROforum is a collaboration between eight of Europe’s largest inter-governmental scientific research organisations, which combine their resources, facilities and expertise to support European science in reaching its full potential. As part of its education and outreach activities, EIROforum publishes Science in School.
Resources
- For an interview with Paul Nurse’s colleague Tim Hunt, see:
- Gebhardt P (2007) Eyes on the horizon, feet on the ground: interview with Tim Hunt. Science in School 6: 9-13.





