Moringa: the science behind the miracle tree Understand article
Moringas have long been known as miracle trees. Now scientists are investigating their properties in depth, as Sue Nelson and Marlene Rau report.
© WEDC, Loughborough
University
In the foothills of the Himalayas grow trees, five to ten metres tall, with clusters of small oval leaves and delicately perfumed cream-coloured flowers. These are Moringa oleifera – the most widely cultivated of the 14 species of the genus Moringa, known as ‘miracle trees’.
“It is called a miracle tree because every part of the tree has benefits,” says Balbir Mathur, president of Trees for Life Internationalw1, a US-based non-profit organisation that provides developmental aid through planting fruit trees, moringas among them. “The roots, leaves, bark, parts of the fruits and seeds – everything. The list is endless.”
Reports in the press about the miraculous nature of the tree may be exaggerated, but it does have some truly impressive properties. Native to northern India but now found widely in Asia, Africa and Latin America, moringas have been used in villages in developing countries for hundreds of years, their uses ranging from traditional medicine, food and cooking oil, to natural pesticide, domestic cleaning agent, and – the latest addition – biofuel.
to a powder before use
© WEDC, Loughborough
University
Moringas are extremely hardy, known in parts of Africa as nebedies, meaning ‘never-die trees’, because they grow on marginal soils, regrow after being chopped down, and are one of the few trees that produce fruit during a drought.
It is yet another useful property of Moringa oleifera, though, that is exciting scientists: when crushed, moringa seeds can help purify dirty water. This could save lives: the World Health Organization estimates that unsafe water, poor sanitation and inadequate hygiene cause about 1.6 million deaths a year globally.
Water purification is mainly a two-step process: initially, water is clarified, removing particles such as minerals, plant residues and bacteria. However, since not all particles readily sink to the bottom, coagulating agents are added to help clump the particles together; these clumps can then be removed by filters or sedimentation. The second step is disinfection, to kill those pathogens that still remain, using chlorine compounds, ozone, hydrogen or ultraviolet light.
Moringa oleifera can help with the first purification step – not only in the developing world but also in the developed world. In industrial water treatment plants, the most prevalent coagulating agents used today are aluminium salts. Most particles that need to be removed from water are charged, so coagulating agents are usually ions; because the coagulating efficiency increases with the square of the coagulating agent’s ionic charge, polyvalent ions such as aluminium are very efficient. However, there is concern – albeit controversial – that long-term exposure to aluminium may be associated with the development of neurodegenerative diseases. Iron salts are an alternative, but they are more difficult to use, as their solubility changes with pH.
and cut open (right)
© WEDC, Loughborough
University
Further kinds of coagulating agents include synthetic polymers, but, as with the other coagulants, the sludge formed in the clarification process needs to be disposed of: so even though synthetic polymers solve the problem of the putative link to neurodegenerative diseases, their lack of biodegradability is an issue.
Since M. oleifera is both non-toxic and biodegradable, and reported reductions in the cloudiness, clay and bacterial content of water after the application of M. oleifera seeds rival the efficiency of aluminium salts (see Ghebremichael et al., 2005), it seems to be a viable alternative.
In certain rural areas of Sudan, women already use M. oleifera to purify water: when collecting water from the River Nile, they place the powdered seeds in a small cloth bag with a thread attached. This is then swirled around in the bucket of turbid water, until the fine particles and bacteria clump together with M. oleifera powder, sinking and settling to the bottom. For drinking water though, the water needs to be purified further – by boiling, filtering through sand or placing it in direct sunlight in a clear bottle for a couple of hours (solarising; see Folkard et al., 1999). You can try a similar technique yourself in class (see box).

Images courtesy of Dr Majority Kwaambwa, University of Botswana
Although a successful pilot study was performed at Thyolo water treatment works in Malawi in 1989-1994 (see Folkard & Sutherland, 2002), developing future industrial treatment methods from M. oleifera relies on knowing exactly what processes take place during the purification. Researchers already know that the active ingredient in the seeds is protein, which accounts for 30-40% of the seeds’ weight. There are at least two proteins that may be active: they are water-soluble and quite small, about 6-16 kDa, so they can readily diffuse out of the cloth bags. At higher concentrations, they aggregate even in solution due to their substantial hydrophobic regions. The protein adsorbs onto contaminant particles, which then clump together and can be separated and extracted.

Image courtesy of Dr Majority
Kwaambwa
But how exactly does this clumping work? Scientists from the University of Uppsala, Sweden, and the University of Botswana in Gaborone, Botswana, set out to investigate further (see Kwaambwa et al., 2010). They produced a purified extract of all the water-soluble protein from the seeds to study how the protein adsorbs onto an interface between water and silica (silicon dioxide, SiO2) as a model for the interface between water and mineral particles.
The team used a neutron beam at the Institut Laue Langevinw2 in Grenoble, France, in a technique called neutron reflectometry, to measure the thickness, density and coarseness of the forming protein layer.
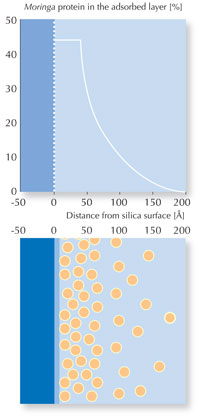
M. oleifera protein is packed
very densely, in a layer about
two molecules thick (50 Å).
Then, with increasing
distance from the silica
surface, the concentration of
adsorbed protein decreases
rapidly.
Click to enlarge image
Image courtesy of Maja
Hellsing
How does this technique work? When you see a layer of petrol on a puddle, you can see a variety of iridescent colours: light bounces off both the top and bottom of the petrol layer. The reflected light waves will be slightly out of phase, and depending on the thickness of the petrol layer, will either add up or cancel each other out, resulting in different colours. Many more materials are transparent to neutrons than to light, and neutron wavelengths are also about one thousand times shorter (0.2-2 nm) than those of light (about 0.5 µm), which is why a neutron beam can be used to measure layers of protein a single molecule thick.
The ‘white’ neutron beam is shone onto the sample, and reflectivity is measured as a function of neutron ‘colour’ (i.e. wavelength), telling scientists how many molecules thick the layer is, how densely packed the molecules are, and how rough the surface of the layer is.
In the M. oleifera experiment, the scientists found that the seed protein forms dense layers thicker than a single molecule even at concentrations as low as 0.025 wt% – so the binding is very efficient. The surface of the layers is remarkably smooth, but the array of M. oleifera protein is not uniform: further away from the silica surface, the number of water molecules among the protein increases, which can be seen as a change in density, as measured by neutron reflection (see diagram, left).
This suggests that the clumping is so efficient because M. oleifera protein has a strong tendency to bind both to mineral surfaces and to other M. oleifera protein molecules, even at very low protein concentrations, due to hydrophobic regions and to the fact that, even when the overall protein is electrically neutral, different subgroups of opposite charge will be ionised.
Work on M. oleifera proteins continues, to develop a non-toxic, biodegradable water purification treatment for which materials are available locally and at a much lower cost than aluminium salts. Questions being addressed include how much seed protein is needed, whether other proteins or biopolymers are suitable, and if other impurities in water, such as natural detergents, affect the action of the process.
Mathur welcomes the scientific scrutiny. “We feel that the moringa tree is very important and needs to be brought to the attention of scientists who can do further research,” he says. “It is not widely known in the Western world yet because it doesn’t grow there.” In the future, the miracle tree could live up to its name. “The moringa could save millions of lives around the world for years to come,” states Mathur. “I cannot emphasise enough how important it is.”
Cleaning water with moringa seeds
Seeds of the Moringa oleifera tree are cheaply available online, as the tree is grown for decorative purposes.
Water will require different amounts of M. oleifera powder to purify it, depending on the impurities present. Around 50-150 mg of ground seeds treat one litre of water: as a rule of thumb, powder from one seed will be sufficient for one litre of very turbid or two litres of slightly turbid water. Experimenting with small amounts of water in a jar will help you work out the correct amount of powder and the optimal stirring times.
You may want to compare the water quality achieved with M. oleifera seeds to that achieved with other methods (see Mitchell et al., 2008, for an example of a different water purification method), and run a competition for the most efficient method of water purification.
- Remove the seeds from the dried pods, if still present, and shell them, leaving a whitish kernel. Discard any kernels with dark spots or other signs of damage.
- Crush the seed kernels to a fine powder and sieve them (0.8 mm mesh or similar).
- Add the powder (approximately 2 g) to one cup of clean water, pour into a bottle and shake for 5 minutes.
- Filter the mixture through a clean cloth into a bucket of dirty water that is to be treated.
- Stir the water quickly for 2 minutes and slowly for 10-15 minutes (do not use metal implements, since this may re-introduce unwanted metal ions removed by M. oleifera). During the slow mixing, the fine particles and bacteria will begin to clump together and sink and settle to the bottom of the bucket.
- Cover the bucket and leave it undisturbed until the water becomes clear and the impurities have sunk to the bottom. This may take up to an hour.
- The clean water may be siphoned or poured off the top of the bucket or filtered through a clean cloth. The process removes at least 90% of the bacteria and other impurities that cause turbidity.
Notes:
Both the seeds and the seed powder can be stored, but the paste (made in step 4) should be freshly made every time water is to be purified.
For safety reasons, water purified in class must not be used as drinking water.
References
- Folkard G, Sutherland J (2002) Development of a naturally derived coagulant for water and wastewater treatment. Water Science and Technology: Water Supply 2(5-6): 89-94
- Folkard G, Sutherland J, Shaw R (1999) Water clarification using Moringa oleifera seed coagulant. In Shaw, RJ (ed) Running Water: More Technical Briefs on Health, Water and Sanitation pp 109-112. Rugby, UK: IT Publications. ISBN: 9781853394508
- Ghebremichael KA et al. (2005) A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Research 39: 2338-2344. doi: 10.1016/j.watres.2005.04.012
- Kwaambwa HM, Hellsing M, Rennie AR (2010) Adsorption of a water treatment protein from Moringa oleifera seeds to a silicon oxide surface studied by neutron reflection. Langmuir 26(6): 3902-3910. doi: 10.1021/la9031046
- Mitchell WA et al. (2008) Science for the Next Generation: activities for primary school. Science in School 10: 64-69. www.scienceinschool.org/2008/issue10/nextgeneration
Web References
- w1 – Trees for Life International provides an international forum on beneficial trees and plants, and has been promoting the moringa tree for many years, sending literature and information to universities, embassies and heads of state, as well as producing educational material for schools. See: www.treesforlife.org
- w2 – To learn more about the Institut Laue-Langevin, see: www.ill.eu
Institutions
Review
This is a thought-provoking article that uses theoretical science (binding abilities of ions, neutron reflection techniques) to explain a real-life situation (using seeds to purify water).
Ideas from this article could be used with students of all ages to carry out some innovative practical work, with appropriate risk assessments. M. oleifera seeds can be bought via the Internet, if you are not lucky enough to have them grow locally, and their effects on clarifying water can be compared to those of other seeds. Are M. oleifera seeds really much better? Younger students could have a lot of fun grinding up different seeds and coming up with different ways of measuring how clear their dirty water becomes.
Older students could take this further and carry out investigations linked to using different parts of the seeds and relating this to seed biochemistry, or compare seeds treated in different ways, e.g. dried versus fresh. Researching the scientific basis of the seed-driven clarification process would certainly be challenging for the most able students.
The article fits well into a number of curricular topics: biology of seeds / biochemistry of extracts; chemistry of water purification / physical behaviour of the salts; physics – perhaps something to do with investigating density of layers – and looking at alternative methods. The idea could form the basis of a cross-curricular project with social science lessons, because of the good links with sustainability and renewable resources.
The article is suitable as a comprehension exercise for students aged 16 and older. Possible questions to ask in a biology lesson include:
- Moringa oleifera is just one of 14 species of the ‘miracle tree’. Which part of the scientific name gives the genus, and which the species?
(Answer: genus: Moringa; species: Moringa oleifera) - In this article, a variety of uses are given for the moringa, including medicine, food and cooking oil. Suggest which parts of the tree might be used for which of the given uses in the article.
(Answers need not be correct, as students are not expected to know, but they should be reasonable suggestions, e.g. oil from seeds; medicine from any part; food from fruit; pesticide from leaves; cleaning agent from roots.) - Moringas ‘regrow after being chopped down’ and are also described as being ‘one of the few trees that will produce fruit during a drought’. Suggest what adaptations these trees might possess in order to do this.
(Answer: again, students would not be expected to know the answer, but could come up with possible ideas such as new trees growing from seeds that were dispersed before the tree was cut down, or the chopping down acting as a kind of coppicing, causing more branches to grow. Being able to produce fruit during a drought could be due to an extensive root system (deep and / or wide, like those of cacti), to leaves that reduce the transpiration rate, or to mechanisms for absorbing dew, such as shallow roots.)
The article can also form the basis of discussion. Possible topics include:
- The evidence for / against aluminium being implicated in neurodegenerative diseases
- The more unusual uses of plants
- The role of the media in scaremongering the public on flimsy scientific basis (such as the drop in sales of aluminium saucepans, after it was proposed that they may be harmful)
- Citizenship topics and / or theme days based on water availability around the world.
Sue Howarth, UK





