Monastic ink: linking chemistry and history Teach article
One of the many purposes of science is to support the humanities. With this in mind, Gianluca Farusi and his students set out to investigate and prepare iron-gall ink, a historically significant material for the transmission of knowledge.
The historical setting

Many mediaeval miniatures of St. John of Patmos demonstrate the importance of ink: they portray the Devil attempting to steal the saint’s precious ink. In the Middle Ages, two kinds of black ink were generally used: carbon ink (a suspension of carbon, water and gum) and iron-gall ink (obtained from oak galls). Carbon ink was used as early as 2500 BC whereas iron-gall ink was used from the 3rd century AD onwards, by individuals such as Leonardo da Vinci, Johann Sebastian Bach, Rembrandt van Rijn and Vincent van Gogh. According to recent research, traces of iron-gall ink have been found on the Dead Sea scrolls and on the lost Gospel of Judasw1.
The reaction that forms the ink pigment was not used in the ancient world to produce ink, but it was known: in his Naturalis Historia (Natural History), Pliny the Elder (23 AD – 79 AD) describes how to distinguish verdigris [Cu(CH3COO)2.2Cu(OH)2], used to process leather, from the cheaper copperas (FeSO4.7H2O) with which it was often adulterated. He writes:
“ …The fraud may be detected using a leaf of papyrus which has been steeped in an infusion of nut-galls: it immediately turns black when adulterated verdigris is applied …”.
Although he could see the transformation, he did not understand it. Now, we know that this ancient test relies on the reaction between the ferrous cation (iron(II)) and gallotannic acid that is at the root of the iron-gall ink preparation (see below).
The educational context
When one of my chemistry students asked me what kind of ink mediaeval monks used, I was inspired to develop a project on monastic ink (see box). In addition to offering an instructive slant on science, it emphasises the links between science and the humanities.
We decided to use the recipe listed by the Venetian Pietro Canepario from his De atramentis cuiuscumque generis (All Kinds of Ink; 1619). In rhyming vernacular Italian, he describes the composition by weight of the ink: “Una, due, tre e trenta a far la bona tenta” (“with one, two, three and thirty parts try to do it well”). Now we know that this ratio of gum arabic, ferrous sulphate, galls and water is not the best for iron-gall ink: because it is too acidic, it eats into the paper over time. However, my goal was not to prepare the best possible ink, but rather to encourage my students to investigate history by using chemistry and to understand the cultural and educational roles of chemistry.
Next, the students investigated galls, and set out to collect common types – oak (both acorn and marble) galls and cypress galls – to allow us to compare three different inks.
We wanted to determine the best type of ink, but according to what criteria? We decided not to stray too far from Canepario’s time, and referred to De subtilitate (The Arcane Links Between Things) and De rerum varietate (The Variety of Things) by Gerolamo Cardano (1501-1576). According to Cardano, a good ink flows well, and is thick, black and bright. We tested our inks, writing with both Pasteur pipettes and pen nibs, and found that acorn-gall ink best fitted these criteria. My personal favourite, however, was cypress-gall ink: although it is less black than acorn-gall ink, it smells wonderfully of resin.
Of course, we ended with a discussion of the chemistry: we considered the reactions taking place at each stage of our ink production, as well as subsequent reactions between the ink and paper, their significance for historical documents and how further damage could be prevented.
The students loved the project, particularly the interdisciplinary and historical aspects. One student remarked that chemistry “seems to be the clue to both modern and ancient puzzles”.
Iron-gall ink ingredients

Image courtesy of Gianluca
Farusi

Image courtesy of Gianluca
Farusi

Image courtesy of Gianluca
Farus
The first certain reference to iron-gall ink is in Martianus Minneus Felix Capella’s book De Nuptiis Philologiae et Mercurii et de septem Artibus liberalibus libri novem (On the Wedding of Philology and Mercury and of the Seven Liberal Arts in nine books; 420 AD), which mentions a mixture of galls and gum. Although other recipes have been preserved, all agree on the basic ingredients: galls, ferrous sulphate (copperas), water and gum arabic.
Galls
Galls are abnormal, induced growths found on the leaves, stems, flowers or roots of plants. The gall-forming agent, often a fly or wasp, deposits an egg into the young plant tissue, and the gall grows around the larva, which feeds and develops within the protective growth. Secretions from the larva, including saliva and excreta, are believed to control the development of the gall.
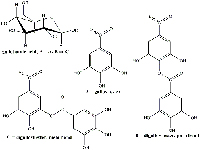
The chemical composition of galls varies depending on the gall-forming agent and the plant in question. The Aleppo gall is particularly rich in tannic acid (65%) and gallic acid (2%); the Bassorah gall (also known as the mad apple of Sodom) contains 26% tannic acid and 1.6% gallic acid; whereas the Acorn gall contains 45-50% tannic acid. All three also contain high concentrations of gallotannic acid. In our project, we used acorn and marble galls (typical oak galls), as well as cypress galls (Figures 1, 2, 3).
All three types of gall can be found across Europe but anything containing tannins could also be used to produce the ink; tea, for example. Alternatively, you could use tannic acid.
Ferrous sulphate or copperas
In ancient times, copperas (FeSO4.7H2O) was extracted by evaporating water from ferrous earths. Later, at the end of the 16th century, it was produced by adding sulphuric acid to iron nails. We used ferrous sulphate, a common substance in the chemistry laboratory.
Water
Since tap water may contain impurities (such as chlorine) that alter the ink quality, we used distilled water. Historically, rainwater was probably used.
Gum arabic
Gum arabic, a natural gum extracted from acacia trees and used as a food stabiliser, maintains the quality of the ink in three ways:
It keeps the iron complex (the pigment) in suspension
It thickens the ink and prevents it from flowing too fast from the pen
It reduces the speed at which the ink soaks into the paper, giving a better and longer-lasting stroke.
Materials and method
Galls, 3 parts by weight
Water, 30 parts by weight
Ferrous sulphate, 2 parts by weight
Gum arabic,1 part by weight

Image courtesy of Gianluca Farusi

Image courtesy of Gianluca Farusi

Image courtesy of Gianluca Farusi

Image courtesy of Gianluca Farusi
- Break the galls into pieces, then grind them in a coffee mill (Figure 4).
- In a beaker, add the water to the ground galls. Leave the mixture to ferment in a sunny corner at room temperature for 3 days.
- Filter the mixture (Figure 5) and add the ferrous sulphate to the solution. Stir well and leave for 3 days.
- Add the gum arabic, stir the mixture and you have your ink (Figure 6). Time to start writing (Figure 7)!
The chemistry involved
Although the reactions are easy to perform, the chemistry involved is rather complex. This experiment works well if younger students (ages 14-15) do the laboratory work, after which older students (ages 17-18) explain to them the chemistry and biology involved.
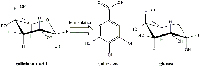
After the galls have been broken up, the reactions occur in two stages: the fermentation of gallotannic acid to gallic acid followed by the formation of the ink pigment.
Galls contain large amounts of gallotannic acid (Figure 8) but relatively little of the gallic acid needed to make the ink pigment. In the fermentation stage, the galls release the enzyme tannase from the Aspergillus niger and Penicillium glaucum fungi that are found in the galls. Over three days, tannase catalyses the hydrolysis of gallotannic acid to gallic acid and glucose (Figure 9).
glucose molecule, the hydroxylic groups
of which are esterified by a mixture of
gallic, digallic and polygallic acids. Click
to enlarge image.
Image courtesy of Gianluca Farusi
Click to enlarge image.
Image courtesy of Gianluca Farusi
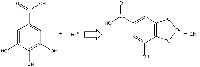
cations react to form ferrous gallate.
Click to enlarge image.
Image courtesy of Gianluca Farusi
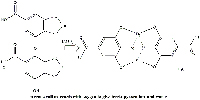
with oxygen to give ferric
pyrogallate and water.
Click to enlarge image.
Image courtesy of Gianluca Farusi
The ink pigment is formed in two further stages: a Lewis acid-base reaction followed by a redox reaction.
Gallic acid and ferrous sulphate form ferrous gallate (a colourless water-soluble compound; Figure 10), plus , H3O+ and SO42-.
Almost immediately, the ferrous gallate reacts with oxygen to produce water and ferric pyrogallate, a black insoluble octahedral complex in which the ligands of each ferric cation are two molecules of gallic acid (Figure 11).
The ink pigment is ferric pyrogallate. Owing to the presence of H3O+ ions, the solution is acidic.
Long-term damage
Iron-gall ink may have been used for 1800 years, but it does not withstand the test of time well. Over the course of centuries, the ink fades, and discolours and damages the paper. This is due to an excess of ferrous ions: the 2:3 ratio of ferrous sulphate to galls is stoichiometrically incorrect, with more ferrous sulphate than is necessary to react with the gallic acid.
Some of these surplus cations oxidise to form Fe3+ oxide which, as it is paler than ferric pyrogallate, fades the original black colour of the ink. Other excess cations catalyse a damaging chain reaction:
Fe2+ + O2 + H+ → Fe3+ + HOO•
Fe3+ + HOO• + H+ → Fe3+ + H2O2
Fe2+ + H2O2 → Fe3+ + HO• + OH– (Fenton reaction)
RH + HO• → R• + H2O
R• + O2 → ROO•
ROO• + R1H → ROOH + R1•
R1• + …..
where Fe2+ is a surplus ferrous cation, O2 is atmospheric oxygen and R is a cellulose moiety.
Furthermore, when an HO• radical attacks cellulose (RH) in the paper, it breaks many bonds and holes appear. In turn, the resulting cellulose radicals (R•) react, forming more cross-links, so that the damaged paper is more closely meshed, less hydrophilic, and consequently drier and more brittle.
Conservation
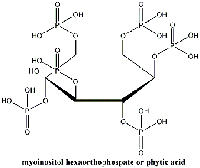
(myoinositol hexaorthophosphate).
Click to enlarge image.
Image courtesy of Gianluca Farusi
One solution to this damage was thought to be ferrous cation chelation: surrounding the ferrous cations with appropriate ligands markedly reduces their oxidation to ferric cations, preventing further damage. However, finding a suitable chelating agent was a challenge: EDTA, for example, does not reduce the oxidation rate of Fe2+.
Recent research at the Netherlands Institute for Cultural Heritagew2, however, offers hope. A treatment based on phytic acid (myoinositol hexaorthophosphate; Figure 12), a natural antioxidant in seeds, leads to the formation of an Fe2+-phytic acid complex (Botti et al., 2005). This inhibits the Fenton reaction, a step in the chain reaction described above, and prevents further damage to the paper.
Conclusion
“There comes a day when both the time and the painstaking study of many generations will reveal what is now hidden; one life would not be long enough to finish such a boundless research… and so these phenomena will become clear over generations of researchers… There will come a day when our descendants will be surprised that we were so ignorant of such obvious things. Many discoveries await the coming centuries when even the memory of us has faded away. The world would be a really trifling affair if each generation did not find matter to inquire into… Nature doesn’t reveal its secrets in one go.”
Seneca, Quaestiones Naturales, Book VII
This topical passage from Seneca (4 BC – 65 AD) still holds true. I like to think that Pliny would be pleased to see how this generation is able to figure out what he could not.
References
- Botti L, Mantovani O, Ruggiero D (2005) Calciumphytat zur Behandlung von Tintenfraß: Wirkungen auf das Papier (Calcium phytate, a natural antioxidant to counter paper corrosion caused by iron-gall ink). Restaurator 26: 35-45
Web References
- w1 – National Geographic website
- w2 – Netherlands Institute for Cultural Heritage website
Resources
- The Ink Corrosion website
Review
This article provides a novel cross-curricular project linking chemistry and history (with possible links to art, forensic science and biology). The idea of making historically accurate ink is interesting, and relies on relatively simple chemistry.
Such a project could form the basis of an investigation including, for example, a comparison of modern and/or traditional inks. The link to the prevention of ink degradation could further extend any investigation. Overall, this is an interesting article with some useful ideas for exploring advanced chemistry.
Mark Robertson, UK





