On track: technology for runners Understand article
When we watch elite runners breaking world records, we rarely think about the chemistry and physics of the running tracks.

João Havelange in Rio de
Janeiro, which will be used in
the 2016 Olympics
Image courtesy of Andrew
Hecker; image source:
Wikimedia Commons
This year, the Olympic Games will give the world’s top athletes another chance to prove themselves and smash records at the events in Rio de Janeiro, Brazil. Along with their training, fitness and sheer talent, the competitors have another factor to help them succeed: technology.
For the runners, it’s principally the latest track surfaces that will enable them to do their best. Perhaps surprisingly, whereas leisure runners exercising in streets and parks rely on their sports shoes to provide the cushioning and spring needed for each step, for professional runners it’s the track itself that fulfils this role. This fact reflects the gap in performance levels between school or amateur sports practised for fun or fitness, and top-level competitive sports. The energies involved in accelerating, decelerating, jumping and so on are markedly different: an Olympic sprinter running at full speed would probably injure herself on a hard track because of the high impact on her joints. In contrast, a jogger or school student wearing cushioned shoes might find athletic tracks too soft.

Image courtesy of Mondo SpA
Forces in action
Running is in effect a series of controlled impacts on the ground, so the ideal surface should provide enough shock absorption to avoid injury (especially to the ankles, knees and ligaments) at the same time as offering a strong, stable base to allow an athlete to push forward.
Let’s think about the forces between the athlete and the running surface that are involved in running. At each step, the athlete uses her leg muscles (and the friction between the track and the sole of her shoes) to push against the ground. Newton’s third law of motion tells us that:
For every action [force], there is an equal and opposite reaction [force].
This means that as the athlete pushes against the track, the track exerts an equal and opposite force on the athlete, pushing her forward. This is often called the ground reaction force.
Newton’s second law of motion tells us that:
Force = mass x acceleration.
So for the athlete, the greater the force pushing her forward, the greater her acceleration.

Image courtesy of tableatny; image source: Wikimedia Commons
Newton’s second law also helps to explain what happens every time an athlete lands during running. When the foot hits the track, it will decelerate to a stop before leaving the track again. The faster the deceleration, the greater the force of impact on the foot. So the track needs to ensure that the deceleration is slow enough to make the impact bearable, but fast enough to sustain running speed. It is here that specialist materials are needed to produce a track that is neither too soft nor too hard.

Image courtesy of Mondo SpA
Hard and soft materials
In everyday life, we encounter materials of varied consistency: from hard, solid metals to soft, flowing liquids. Let’s think about these characteristics in more detail.
- As well as being hard, metals are elastic materials. Like a spring, a metal wire will stretch when a force is applied and will then return to its original length when the force is removed. (If the force is too strong and stretches the metal past its elastic limit, the wire will be stretched permanently.) Energy is stored by the material when it is stretched and is then quickly released when it springs back.
- Liquids are soft, non-elastic materials. They will flow freely if a force (such as gravity) is applied and will not keep their shape. The mechanical energy is dissipated, rather than stored in the material. Materials of this kind are described as viscous.
As figure 5 illustrates, a purely elastic material (e.g. metal or concrete) will store all the energy of impact and return it instantly. However, this produces ground reaction forces that are not safe for runners: some energy needs to be absorbed by the track material. Viscous surfaces, on the other hand, will absorb the energy of the foot impact but will not give anything back.
Between these two extremes are viscoelastic materials. These can dissipate part of the energy of impact – enough to protect the athlete’s ligaments – while also conserving enough to provide a suitable reaction force to propel the athlete forward.
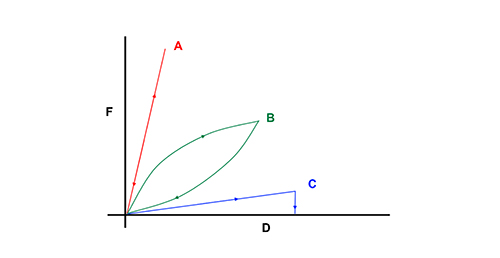
Image courtesy of Joao Bomfim
Why polymers might be the answer
So where can we find a material with the right viscoelastic characteristics? An important group of viscoelastic materials are polymers – the family of materials that includes plastics, rubbers and glues. Polymers are made up of huge molecules comprising hundreds or even thousands of atoms. Because of their size, polymer molecules can interact with each other by lining up and becoming physically entangled. Rubber molecules are special because although they entangle, they do not normally line up with each other, remaining somewhat ‘loose’; they have what polymer scientists call free volume. This means that the molecules can bend and move, sliding away from or towards each other, allowing the material to stretch.
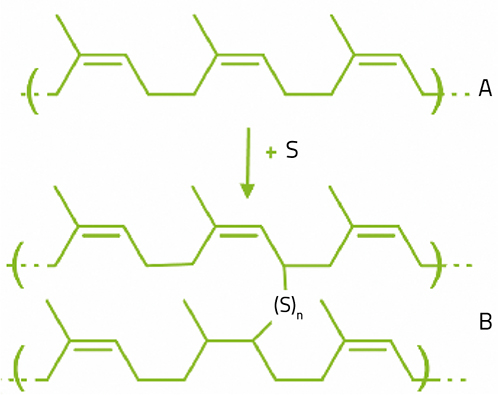
reacts with sulfur (S),
resulting in vulcanised
rubber (B)
Image courtesy of Joao Bomfim
Besides physical interactions, chemical (covalent) bonds called crosslinks can form between polymer molecules. Polymer materials with many crosslinks are usually hard: epoxy glue is an example of this. In contrast, most rubbers have a relatively small number of crosslinks, so they are softer. Rubber can be hardened by a process called vulcanisation, in which sulfur atoms form additional crosslinks between molecules (figure 6).
If the free volume allows rubber to be stretched, the forces and entanglements between the polymer molecules, especially the crosslinks, will pull them back to their original position, giving rubber its elasticity.

waiting to be recycled or
incinerated
Image courtesy of Mark
Buckawicki; image source:
Wikimedia Commons
The job of the polymer chemist is to know how to choose the right materials and processing conditions for a given use, such as running tracks. Beyond choosing the right rubber with the appropriate number of crosslinks, a polymer chemist must also pay attention to the right antioxidants, so the tracks will not degrade. The heat and UV light from exposure to sunlight and outdoor weather are known to promote chemical reactions. This is because the process of rubber hardening (from vulcanisation, ageing or weathering) never actually stops. Without proper antioxidants to prevent more and more crosslinks forming, a running track would become hard and lose its shock-absorbing properties, behaving more and more like a purely elastic material, until the runners feel as though they are running on concrete.

surface composed of recycled
rubber
Image courtesy of Oxyman;
image source: Wikimedia
Commons
Another important contribution of science to running tracks is linked to environmental concerns and recycling. Indeed, many tracks use recycled rubber in their composition. It is a clever way of reducing the waste that ends up in landfill or is incinerated. Tyres are strong and flexible – they have to support the weight of a car and must not break even if they hit the curb or a hole in the road. Old tyres can be ground into crumbs, cleaned and incorporated into the bottom layer of the track, which is not visible and does not come into contact with the athletes. Tyre rubber has excellent viscoelastic properties and, when combined with new rubber material on the track surface which protects it from oxidation, it can give a safe and fast running track. Indeed, tyre crumb rubber is already used in many playgrounds as a safe, cushioning flooring to protect children when they fall. In much the same way, it protects top athletes while they do their best to break world records. So when you’re watching athletes competing at the Olympic Games this summer, you can appreciate the science behind those gold medals.
Resources
- To learn more about the history of running-track technology, see:
- Lovett RA (2008) The technology of athletics tracks. Cosmos 15 Aug. http://archive.cosmosmagazine.com or use the direct link: http://tinyurl.com/zxhkt9x
- For a video about athletic speed increases and sports technology, see:
- Epstein D (2014) Are athletes really getting faster, better, stronger? TED2014, filmed March. www.ted.com or use the direct link: http://tinyurl.com/mgkpo7g
Review
Before beginning a traditional lesson about polymers or Newton’s laws, wait a moment and read this article on the technological secrets of modern sports tracks. This concise and simple text contains plenty of interesting information and lots of inspiring ideas for teaching organic chemistry, physics, biology and environmental science. The author appeals to students’ curiosity and interest in sport in the run-up to the 2016 Olympic Games, using clear examples from everyday life.
The article is suitable for science teachers and students at secondary school. It could be used for a warm-up activity before introducing organic chemistry (polymers, chemical structure of natural and synthetic rubber, chemical bonds, vulcanisation, rubber chemical properties and industrial use). It could also be used as a starting point for a lesson on physics (Newton’s second and third laws, elasticity, viscosity, or human body levers), biology (anatomy, muscular contraction, or biomechanics) or environmental science (the use of rubber, rubber production, manufacturing, natural and synthetic rubber, rubber life cycle, disposal and recycling). Another interesting discussion topic could be the technology of facilities for professional and amateur sports. It could also be used as a starting point for an investigation into the technical details of sports equipment such as shoes, balls, swimsuits or helmets.
Possible comprehension questions include:
- Which body parts are NOT commonly injured by the impact with the ground when running?
- Ankles
- Toes
- Ligaments
- Knees.
- Compared to tracks for amateur sporting events, professional running tracks are:
- Softer
- Harder
- More elastic
- Similar.
- Which of the following statements about rubber molecules is false?
- They are huge polymers
- They are physically entangled
- They have a free volume
- They line up with each other.
Giulia Realdon, Italy





