The magic sand mystery Teach article
Using an everyday toy can introduce mystery into the classroom and help explain chemistry.

Image courtesy of Johanna
Dittmar
How do you make a sand castle when your sand cannot get wet? In this activity, students between the ages of 12 and 16 spend 2–3 hours investigating magic sand, a well-known toy, to understand its chemical properties. This challenge leads students to investigate hydrophobicity and surface chemistry using the five phases of inquiry-based learning: engage, explore, explain, extend and evaluate.
What is magic sand?
Magic sand (also known as hydrophobic sand) is mainly known as a gimmick that you can buy in toy stores or via the Internet (figure 1), but chemistry teachers have known about it for many years and the science behind the toy has a range of real-world uses.

minimise contact with magic
sand
Image courtesy of Steve
Jurvetson; image source:
Wikimedia Commons
Unlike normal sand, magic sand has a hydrophobic surface that repels water, so it does not get wet. Instead, magic sand clumps together under water and behaves unlike normal sand. Although some versions are sold as a toy, hydrophobic sand is also produced industrially and is used for water sealing (e.g. foundations) or for collecting oily impurities or small spills. Another intriguing application can be found in pet stores under the brand name Kit4Cat. This product is designed to collect cat’s urine for medical examinations in a way that the cat finds less stressful than the alternative of a catheter. Many videos on magic sand can be found on Youtubew1.
One way to create magic sand is to treat normal sand with trimethylsilanol ((CH3)3SiOH) vapour. The trimethylsilanol forms covalent bonds with the hydroxyl (–OH) groups on the sand molecules’ surface, replacing the hydrophilic –OH groups with hydrophobic siloxaneones.
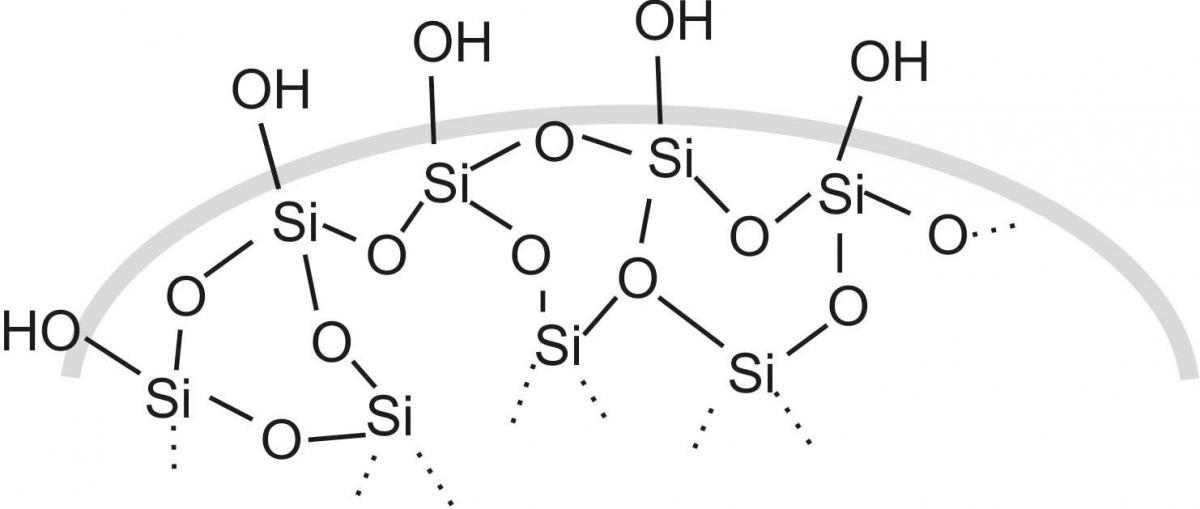
surface of regular sand
Image courtesy of Ingo Eilks
Another method is to coat the sand particles with extremely thin layers of hydrophobic material such as wax, resin, bitumen or plastic. In this method the hydrophobic coating forms intermolecular interactions (not covalent bonds) and is thus less resilient to hydrophobic solvents (such as acetone). The latter method is more economical and if you cannot find magic sand to buy, you can make your own using normal sand and a waterproofing spray.
The mystery of the magic sand
Within the context of the science-education project TEMI (Teaching Enquiry with Mysteries Incorporated), we invented a story to be used in the ‘engage’ phase to increase students’ interest by creating a personal context and making learning more meaningful. The TEMI team in Israel proposed the story of James (see box).
Materials
Each student, or group of students, will need:
- One tablespoonful (15 ml) of normal sand
- One tablespoonful (15 ml) of magic sand
- 50 ml of water
- Two 100 ml beakers
- Two droppers.
Students may also request different solvents such as oil, acetone, ethanol, hexane or liquid soap.
Safety note
Some of these solvents are highly flammable and care should be taken to follow local safety regulations. See also the general Science in School safety note.
Procedure
- Introduce the students to the mystery of magic sand by telling the story of James (see box below). This is the ‘engage’ phase.
- Give the students a sample of the normal sand, and a sample of magic sand, putting each into a 100 ml beaker.
- Provide water and droppers and ask the students to build sandcastles with both samples by wetting the sand. Alternatively, the teacher can demonstrate the behaviour of the magic sand, as shown onlinew1.
- Based on the story, the students will start to compare the magic sand with the normal sand. During this ‘explore’ phase (see the information box for learning phases), the students investigate the behaviour of the magic sand using water and various other liquids, thus developing a feeling for the differences between hydrophilic and hydrophobic substances.
- Provide access to the other liquids suggested above so that the students can see how the two types of sand might react to them.
James’s story

Rochon; image source:
Wikimedia Commons
James is an old man, but ever since kindergarten he has been crazy about building sand castles. Over the years, they got bigger and bigger, more detailed and more elaborate: he would make fairy-tale figures, animals and buildings. James became a champion sand-castle builder but one day he entered a competition that was a little different. He went to the beach and was assigned a small patch of sand. He took a bucket of water and poured it over the sand, but something really strange happened: the sand would simply not get wet. How can you build a sand castle when the sand won’t get wet?
At first James was convinced he would lose the competition. But in the end he was able to build a sand castle.
Now it’s your turn. James gave me a sample of the sand that I can give you. How do you think he managed to build a sand castle? Can you?
What happens?
In the ‘explain’ phase, help the students to learn about intermolecular interactions, especially hydrogen bonds. Normal sand can form such bonds, since the surface is polar (see figure 2), and so the water creates a connection between the sand grains that can hold the grains together.
Magic sand is different. The surface of the grains of magic sand is hydrophobic (apolar), so hydrogen bonds cannot form between the sand particles or with water molecules. The sand repels water, but can be ‘wetted’ by nonpolar liquids, such as vegetable oil, which form van der Waals interactions with the magic sand’s surface. This would of course be one solution to the problem of building a sand castle with the magic sand.
Idea for a follow-up activity
In the ‘extend’ phase, the students can search for optimal ways of building a sand castle. They may be encouraged to ask questions and to design their own experiments to answer them, for example, what is the best solvent to use and at what ratio? They can also investigate the technical applications of hydrophobic sand, as briefly mentioned in this article in the ‘evaluate’ phase. Students could then write a report on the magic sand and its chemistry and uses or create a useful guide on how to use magic sand.
The TEMI project
This activity was developed as part of the Teaching Enquiry with Mysteries Incorporated (TEMI) project, an inquiry-based science-education (IBSE) project funded by the EU under the 7th Framework Programme from 2013 to 2016. TEMI provides science teachers with tools needed to teach IBSE using unexpected and surprising phenomena and by implementing an innovative model for inquiry learning.
IBSE focuses on student inquiry as the driving force for learning. Teaching is organised around questions and problems in a student-centred process and students learn through and about scientific inquiry rather than by teachers presenting scientific content knowledge.
The problem-solving process in TEMI is based on the 5E model of inquiry learning, which structures the learning process into five phases: engage, explore, explain, extend and evaluate. This model was proposed in the 1980s in the USA and has since influenced science teaching in many countries (Bybee et al, 2006).
Information on all these aspects, instructions and examples can be found on the project websitew2. The website also contains other media, such as instructions for lesson plan design, videos of mysterious phenomena, and a smartphone app for independent learning about amazing scientific phenomena.
References
- Bybee RW, Taylor JA, Gardner A et al (2006) The BSCS 5E instructional model: Origins and effectiveness. Colorado Springs, CO, USA: Biological Sciences Curriculum Study
Web References
- w1 – Watch the Youtube video of Ran Peleg presenting the Magic Sand Mystery.
- w2 – Further resources can be found on the TEMI project website (available in several languages).
Resources
- For more ideas on using magic sand in the classroom, see:
- Anonymous (2000) Magic sand. Journal of Chemical Education 77: 40A. doi: 10.1021/ed077p40A
- Goldsmith RH (2000) Illustrating the properties of magic sand. Journal of Chemical Education 77: 41. doi: 10.1021/ed077p41
- Vitz E (1990) Magic sand: modeling the hydrophobic effect and reversed-phase liquid chromatography. Journal of Chemical Education 67: 512–515. doi: 10.1021/ed067p512
Review
The article by Ran Peleg and colleagues describes an article from the TEMI project, which aims to teach science using the IBSE approach, starting from little mysteries, magic or myths. The proposed approach is friendly and engaging, but it is also effective in leading students through a rich learning route, where they can explore a methodology similar to those used in real scientific research. Moreover the addressed topics (e.g. chemical bonds, the properties of water molecules, and organic chemistry), which are often perceived by students as heavy and boring, emerge spontaneously as part of the inquiry into the mysteries of magic sand.
For these reasons I recommend the article to science teachers looking for an inspiring way to introduce organic chemistry to students aged 14–18. The web references are also valuable for discovering other resources from the TEMI project and to stimulate teachers to use acting and storytelling in science teaching.
Giulia Realdon, Italy





