Student competition: the search for the strangest species on Earth Inspire article
Get a glimpse into the weird and wonderful life on Earth with the three winning entries in the Science in School writing competition.

In issue 36 of Science in School, we invited students across Europe to enter our first writing competition to tell us about their choice for the strangest species on Earth. More than 80 students submitted their work, but three entries convinced the judges that their chosen organisms – the naked mole-rat, the jellyfish Turritopsis dohrnii, and the freshwater polyp Hydra vulgaris – are indeed the strangest on Earth. Coincidentally, these species all share one unusual feature that evidently sparked many students’ imaginations – they can defy ageing.
We are now pleased to share the winning entries with you, alongside commentaries from scientists working on these organisms.
Age category: 4–10
Naked mole-rat (Heterocephalus glaber)
Hayden Cookson (aged 7), UK
I think the naked mole-rat is the strangest organism because doctors think that it can help to cure cancer. This is because naked mole-rats never get cancer and people think they are resistant. They can move their incisors individually or together like chopsticks.

Hayden Cookson
What the scientist says…
Dr Ewan St John Smith is a senior lecturer in pharmacology at the University of Cambridge and the director of the university’s naked mole-rat initiative, which aims to bring together experts to identify molecular explanations for this species’ highly unusual physiology.
Why do you think the naked mole-rat is so unusual?
Outwardly, naked mole-rats look like cocktail sausages with legs and teeth, but beyond this comical appearance lies a world of fascinating biology that could help scientists identify new treatments for a wealth of conditions.
Naked mole-rats are cold-blooded and live in large colonies (usually about 80 animals) headed by a single breeding female (the queen) – a highly unusual characteristic for a mammal. Naked mole-rats can live for up to 30 years, whereas a similar sized mouse has a maximum life expectancy of only about 3 years. How do naked mole-rats live so long? One reason could be their high resistance to cancer, one of the many unusual extremes displayed by this species.
Naked mole-rats respond normally to mechanical and thermal stimuli, but they do not feel pain in response to acid. Naked mole-rats can also cope for long periods of time without oxygen due to their cells being able to use fructose to power energy production during these times.
How do you use the naked mole-rat for your research?
By understanding the unusual characteristics of the naked mole-rat, we can discover more about normal physiology in other mammals. For example, understanding the genetic mechanism behind the naked mole-rat’s insensitivity to acid has increased scientific understanding of how pain works. If scientists can determine why naked mole-rats are resistant to cancer, it may lead to the development of new cancer treatments. And understanding how brain cells of the naked mole-rat survive without oxygen could result in the development of novel treatments to prevent brain damage in stroke patients, which occurs when the blood flow to the brain is cut off, depriving cells of oxygen and glucose.

Meghan Murphy, Smithsonian’s National Zoo/Flickr
Resources
- Read more about the life history of the naked mole-rat on the Smithsonian’s National Zoo website.
- Learn more about the extraordinary traits of the naked mole-rat in this Wired article.
- The Telegraph reports on how scientists discovered that naked mole-rats can survive for 18 minutes without oxygen.
- Learn more about the University of Cambridge’s naked mole-rat initiative.
Age category: 11–15
Turritopsis nutricula (now classified as Turritopsis dohrnii)
Ana Aragón, Sara Hidalgo, Xavi Valeri (aged 13), Spain
We think that the strangest organism on Earth is Turritopsis nutricula. It’s a hydroid jellyfish of the family Oceanidae. It’s originally from the Caribbean Sea, but now it’s found around the world, in all the warm and tropical seas. Since scientists spotted it in Colombia, it has also been seen near Japan and in the Mediterranean Sea.
It’s about 4–5 mm in diameter. It’s tall with a transparent and gelatinous skin. The young organisms have eight tentacles and the adults can have 80–90 tentacles! It has a big red stomach inside, and it can shine in the dark! Scientists think that it’s because it’s toxic. We think that this species is strange because scientists say that it’s immortal. They think that it’s immortal because it doesn’t reproduce in the normal way.
The reproduction is done in different ways. Some species are sexual but others are asexual. Sexual jellyfish (male and female) drop sperm and eggs into the water allowing them to fertilise and create larvae. Individual larvae search for a place in the sand and when they touch the ground, they become polyps. Each polyp grows and shows some tentacles and then it divides into parts, which float. Then, in the water, they join together with the tentacles facing downwards to create the jellyfish. The jellyfish grows and when it’s older, its cells start to regenerate and it returns to a polyp. It can do this process in all its stages. That means that it’s a species that has a life cycle in which it returns to the polyp phase after it arrives at sexual maturity.
Laboratory studies showed that 100% of the jellyfish specimens could return to the polyp phase. This species (Turritopsis nutricula) is the only jellyfish that has the ability to do it, because to return to the polyp phase, the jellyfish has to have some specific cells. But that doesn’t mean that it is indestructible. It can die, for example, because it has some illness or because some predators eat it. Professionals say that this ability to avoid dying is probably unique in the animal kingdom – that’s the reason why we think this is the strangest organism on Earth.
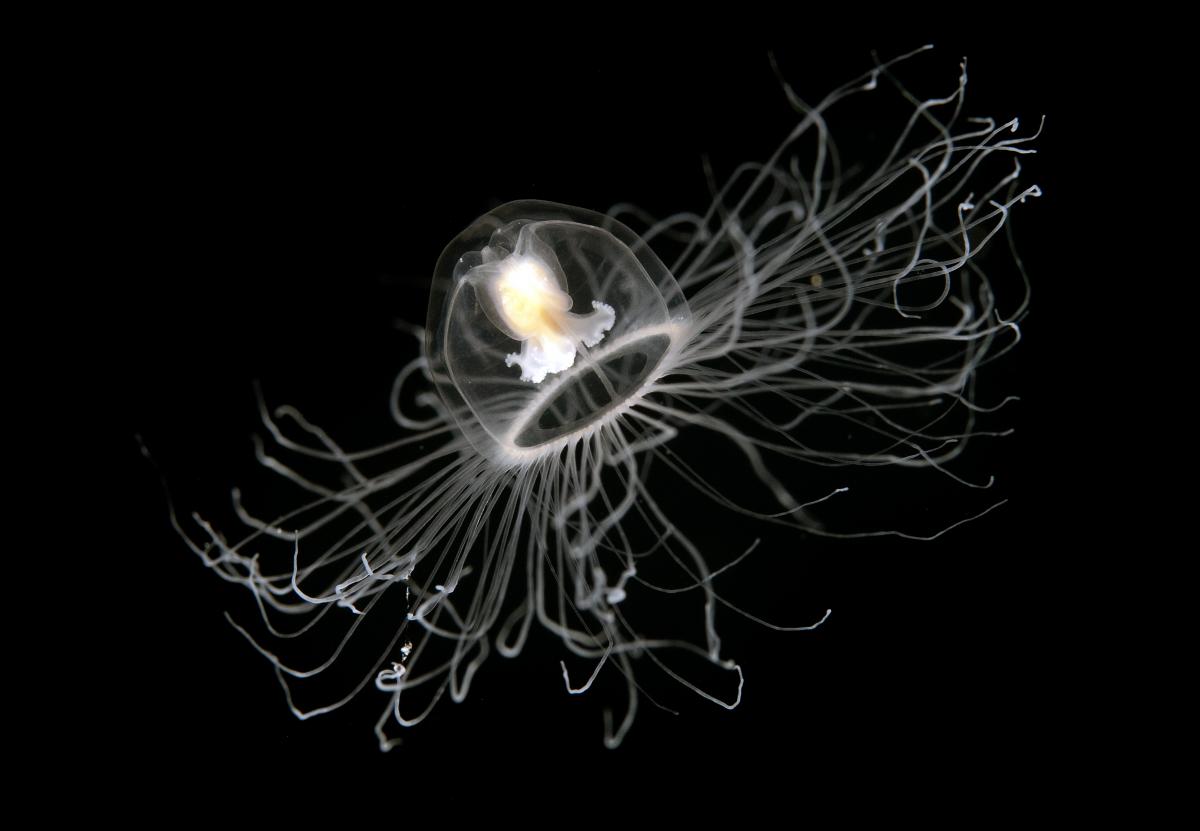
zaferkizilkaya/Shutterstock.com
What the scientist says…
Dr Maria Pia Miglietta is an assistant professor in the department of marine biology at Texas A&M University in Galveston, USA. Her laboratory studies the evolution and genetics of Hydrozoa and jellyfish, including reverse development in Turritopsis dohrnii.
Why do you think Turritopsis dohrnii is so unusual?
T. dohrnii is a species with a very unusual life cycle. Most organisms reproduce, age and die, but T. dohrnii has escaped this fate. When faced with stressful conditions – such as a lack of food, sudden change in salinity, or physical damage – this jellyfish species avoids death by reverting to the polyp stage.
The jellyfish settles on the ocean floor and develops into a cyst-like mass of cells. Around 24 to 72 hours later, this cluster transforms back into a polyp, which grows into a colony that can produce hundreds of new jellyfish. This process is a true metamorphosis, albeit in the opposite direction of the normal developmental cycle, and it has earned T. dohrnii the name ‘immortal jellyfish’.
We believe that the ability to revert to the polyp stage in unfavourable conditions makes this species a good hitchhiker in ballast waters of ships. This helps explain why T. dohrnii has been found in marine waters around the world, including Italy, Japan, Florida, Panama, Brazil and California.
How do you use Turritopsis dohrnii for your research?
My research group studies the unusual life cycle of T. dohrnii from genetic and cellular perspectives. We are trying to understand which genes allow T. dohrnii to avoid senescence, and are particularly interested in what happens in the cyst-like mass of cells at the genetic level. The unique life cycle of Turritopsis may help us understand the biology of ageing, regeneration and cellular differentiation.
Resources
- Learn more about the discovery of the immortal jellyfish and how scientists are studying this species in the article ‘Can a jellyfish unlock the secret of immortality?’ from The New York Times Magazine.
- Watch a video from SciShow explaining how Turritopsis dohrnii extends its life cycle.
- A study in 2008 compared the DNA of immortal jellyfish from waters around the world and found that all the genes that the scientists looked at were identical. Read more about this discovery on the National Geographic website.
Age category: 16+
Hydra vulgaris
Aleksandra Markowska, Halina Ravensdale (aged 17), Poland
Take a close look at your aquarium. Can you see anything special and peculiar?
Looking for the strangest organism on Earth, we spent several days travelling across jungles, snorkelling through depths of oceans and freezing on the North Pole, before we realised that the most bizarre animal might be closer than we had thought. Its ability to regenerate after being cut, the abysmal eating process, and the fact that it does not undergo senescence and appears to be immortal are all proof that Hydra vulgaris can be considered one of the strangest living organisms.
To entirely understand the extraordinary way the animal nourishes itself, we must focus on the exterior of its body. A Hydra vulgaris is usually about 5 mm to 15 mm long, although rarely some can reach up to 3 cm. It has a body form of a polyp. Its simple structure, consisting of a cylindrical main body, ends with several tentacles that serve as a weapon. The cnidae contain nematocytes that have neurotoxins which are released in the form of a dark thread to paralyse small water animals. The other side terminates with a foot called a basal disc used to stick to surfaces. The astonishing thing is that unlike other animals Hydra vulgaris has to transform its body in order to consume a prey. The transformation begins with the deformation of cells. Soon, a black maw is moulded. Following the ‘mouth’ construction stage, the animal sucks in the paralysed victim as the slot closes behind it, shattering any hope of escape. As staggering as it seems, it gets even more peculiar.
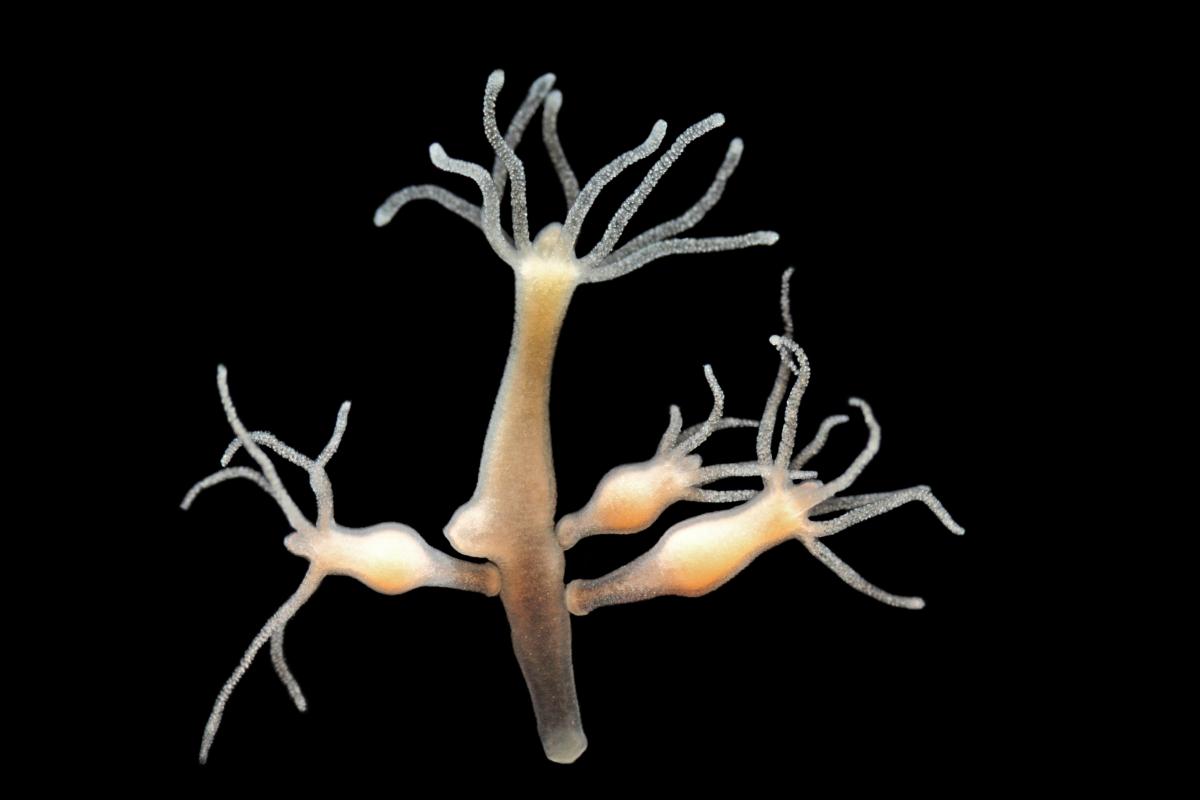
Lebendkulturen.de/Shutterstock.com
Now it is time for an observation. You can test Hydra’s regeneration abilities using a sterile scalpel blade. Cut it into two parts. After several hours, you will notice that wounding its body does not kill it – just like the mythical Larnaean Hydra, it is able to rebuild the segregated parts. This incredible process is called morphallaxis. The animal reorganises its body to form a new smaller version of itself, which is followed by the organism’s growth. When we cut a hydra, the part with the head or foot will transform itself into a whole organism with a new foot or a new head, respectively. A part with neither foot nor head will form a new hydra with both of them. Ethel Browne experimented with transplanting a hypostome and foot to the middle part of another hydra and discovered the existence of the head and basal activator gradient responsible for the formation of a new head or foot when damaged. Soon, the presence of the head and basal disc inhibitor gradient that prevented more than one head or foot from forming was discovered.
It is common knowledge that human beings replace skin cells every three weeks. Imagine how phenomenal it would be if we could exchange all the other cells from our entire body as well at the same time, which would result in immortality. That is exactly what the Hydra vulgaris does – it eludes the whole process of ageing, fertility loss and dying. It is necessary to emphasise the existence of a specific pattern in nature: animals that have offspring sooner, die sooner. Hydrae have progeny after a couple of days, thus they should die at the age of one month but they do not. To profoundly understand this phenomenon, we have to know what is happening at the molecular level. The continuous exchanging of Hydra’s cells is caused by the self-renewal capacity of its stem cells. A research team based in Kiel, Germany, discovered that the FoxO gene (also present in humans) is responsible for maintaining the activity of these cells.
Hydroids could be a salutary examination object in the medical area. Research conducted by a group of students at University College London, UK, found that genes associated with neurodegenerative disease are exactly alike in humans and hydrae. Hydrae possess genes specifically associated with Huntington’s disease and Alzheimer’s disease. Scientists continue to observe hydrae in the belief that they are capable of replacing degenerated genes, thus curing themselves of the disorders mentioned above.
To summarise, we consider these freshwater polyps as the strangest animals on Earth not only because of their bizarre exterior or their weird eating habits, but also because these tiny creatures could assist us in breaking through obstacles that educated doctors cannot overcome with patients who have neurodegenerative illnesses.
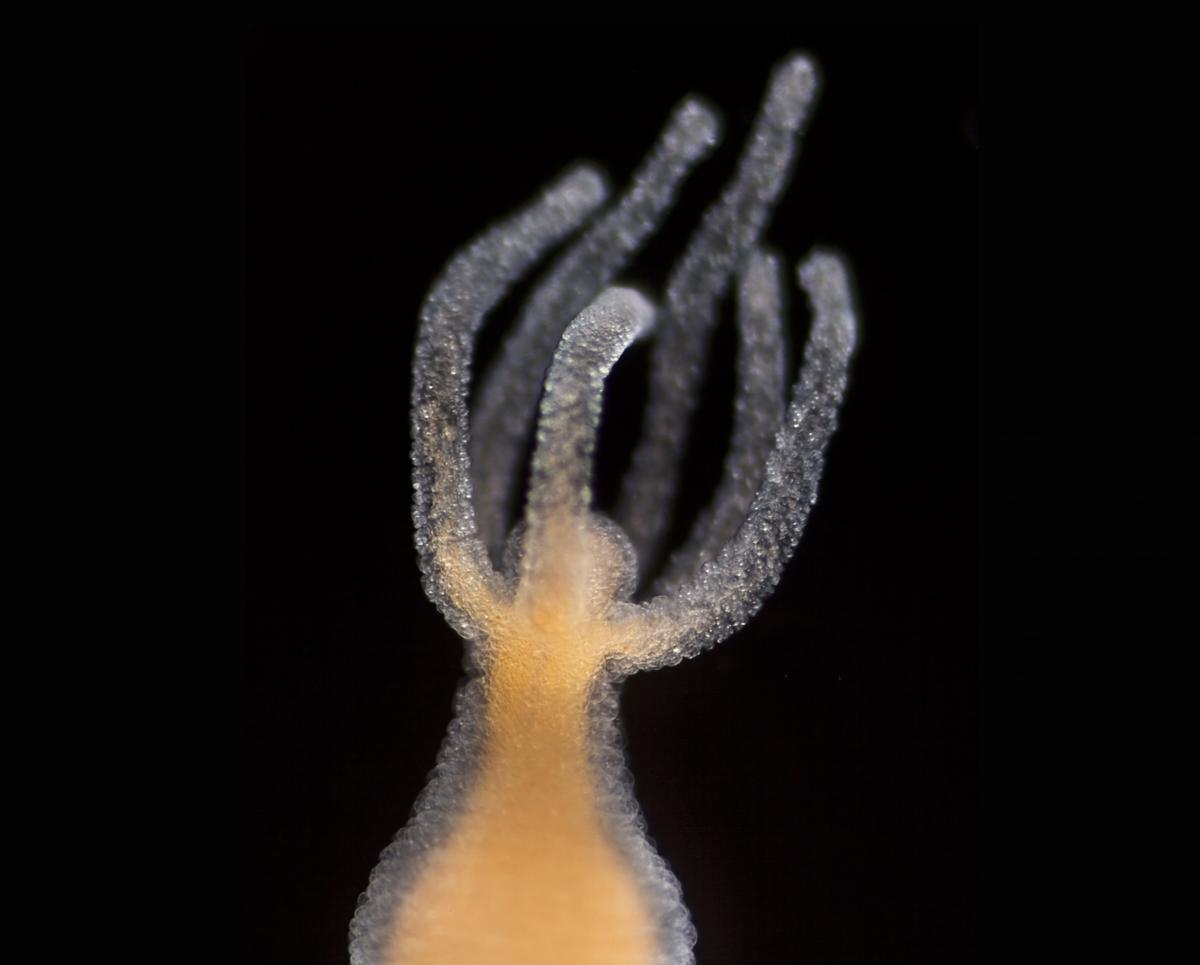
Lebendkulturen.de/Shutterstock.com
What the scientist says…
Dr Eva-Maria S Collins is an associate professor in physics and cell and developmental biology at the University of California, San Diego, USA. Her research aims to use quantitative and biophysical approaches to answer fundamental biological questions in embryonic development and regeneration. For her studies, she works with two fascinating regenerative organisms: freshwater planarians and the cnidarian Hydra vulgaris.
Why do you think Hydra vulgaris is so unusual?
At first glance, the freshwater polyp Hydra vulgaris is a rather inconspicuous animal – you may even mistake it for a plant. But don’t let its appearance fool you – this little creature, just a few millimetres long, has amazing capabilities: cut a piece of its body – or even dissociate the animal into a soup of individual cells – and it will regenerate into a new Hydra. Moreover, one can separate and recombine tissues from different individuals of Hydra, generating chimeric animals with different characteristics.
Since Hydra is such a strong regenerator, it can also reproduce asexually through budding, and purely asexual individuals actually seem to defy ageing altogether. The feeding behaviour of Hydra is also unusual: since they lack a permanent mouth opening, Hydra have to rip a hole in their ‘face’ every time they want to feed.
How do you use Hydra vulgaris for your research?
Hydra’s simple anatomy, optical transparency, ease of manipulation and unusual regenerative capabilities make it an excellent model to study fundamental biological questions. For example, by understanding how Hydra regenerate from re-aggregated cells, we can discover important principles of how cells self-organise, which may also play a role in pattern formation during embryonic development in other organisms.
My research group is particularly interested in how mechanical forces (such as how cells push and pull on each other and their extracellular environment) guide cell behaviour and tissue organisation. If we understand how cell behaviours are regulated, we can use this knowledge to direct stem cell behaviour to repair diseased or damaged tissue – the goal of regenerative medicine.
Resources
- Understand how a Hydra’s cells split apart when it opens its mouth with these videos from EurekAlert! and HowStuffWorks.
- Watch the animation ‘The animal that wouldn’t die’ from Aeon to understand the regenerative power of Hydra vulgaris.
- Read about the role of the transcription factor FoxO in regulating stem cell maintenance in Hydra. See:
- Boehm A M et al (2012) FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proceedings of the National Academy of Sciences USA 109(48): 19697–19702. doi: 10.1073/pnas.1209714109
- Watch the SciShow video about the immortality of Hydra vulgaris and what we can learn from this organism.
- Biologists have sequenced the Hydra vulgaris genome and discovered genes linked with Huntington’s and Alzheimer’s diseases.
- Learn more about the Collins laboratory’s research using Hydra at the University of California.





