Energising enzyme activities Inspire article
Activities you can use again and again, much like enzymes themselves.
Without enzymes, the metabolic processes occurring in all cells would slowly grind to a halt. These macromolecular catalysts are essential for accelerating reactions at rates fast enough to sustain life and they are key to every biological process.
What is the role of an enzyme?
Enzymes, like all catalysts, provide an alternative, faster route to the desired product, resulting in a quicker reaction. The alternate reaction pathway they open up has what we call a lower activation energy, which means that the speed molecules collide doesn’t need to be so high for them to react. All catalysts take part in the reaction that they catalyse but they do not undergo any permanent change at the end of the reaction. However, unlike inorganic catalysts, enzymes are highly specific. They usually catalyse only a single chemical reaction and the enzyme’s active site is shaped specifically for one type of substrate. Using two accessible enzymes, amylase and invertase, this classroom protocol can help students to understand what makes enzymes special, and see how they differ from inorganic catalysts.
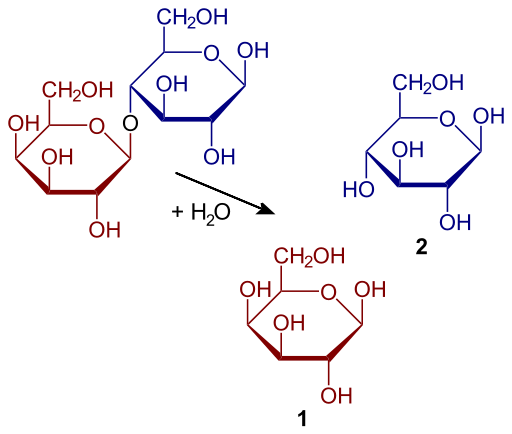
and glucose (2). Image courtesy of
Yikrazuul; image source: Wikimedia Commons
As well as being used in our bodies, enzymes are also used commercially. You will find them in your washing detergent, they are needed to produce wine and beer, and they are even used to make the clothes you wear. Enzymes are often immobilised since this has a number of commercial advantages including allowing the enzyme to be easily removed.
To understand one commercial application, students can follow an activity using immobilised lactase to make lactose-reduced milk. The enzyme hydrolyses the lactose in milk into two smaller sugars, glucose and galactose. Producing lactose-reduced milk is not only a great introduction to enzyme immobilisation, but it is also a chance to highlight that despite cats’ traditional fondness for milk, they are actually unable to digest large amounts of lactose much like 75% of the human population!
How are enzymes constructed?
Despite their huge variety and specificity, enzymes all share an identical structural principle – a central co factor is surrounded by a long biological chain that wraps itself around to create such a specific shape, usually made of protein.
The length of the protein chain in enzymes varies from a few dozen to a thousand amino acids but their tiny structure, measuring only a few nanometres, means that enzymes and other proteins cannot be observed even with the strongest light microscope. To make protein structures ‘visible’ scientists use crystallography.
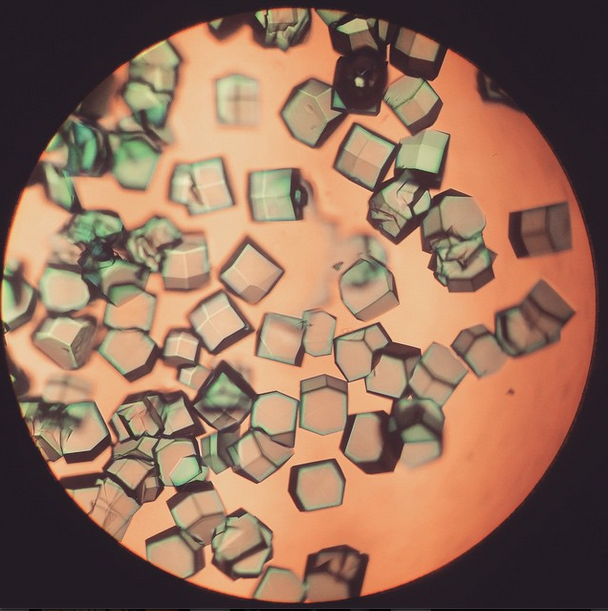
Zanecrc; image source: Flickr
The first enzyme ever crystallised to allow scientists to see just how that chain was arranged was a urease extracted from jack beans. Urease breaks down urea (CH4N2O) into ammonia and carbon dioxide and at home or in the classroom you can use it to visualise enzyme activity. All you need are some soya beans, red cabbage and a few simple chemicals. As ammonia is produced you can detect the pH change with red cabbage indicator.
Another practical activity from our archive focuses on modern x-ray crystallography and investigates the crystallization of a different enzyme called lysozyme, which damages bacterial cell walls. But although x-ray diffraction is the technique that most often comes to mind for examining protein structures, neutron crystallography of enzymes is becoming increasingly useful, especially when designing drugs.
Unlike x-rays, neutrons can locate the positions of all the atoms in the enzyme, including hydrogen. Researchers have recently used this to study the binding between an anti-retroviral HIV drug (amprenavir) and its target enzyme, HIV-1 protease. The researchers found that only two strong, direct hydrogen bonds exist between the drug and the enzyme. This enzyme is key to the life cycle of HIV and drug designers may now be able to strengthen this binding of the drug to the enzyme by subtly modifying the drug’s molecular structure, increasing the effectiveness of the drug and reducing the necessary dosage.





