Supporting materials
Supporting information on the chemistry of fireworks (Word document)
Supporting information on the chemistry of fireworks (PDF file)
Download
Download this article as a PDF

Did you realise that fireworks cause measurable air pollution? Tim Harrison and Dudley Shallcross from Bristol University, UK, explain how to investigate atmospheric pollutants in class.

Whether at New Year, on Guy Fawkes Night or at Diwali, most of us have witnessed a firework display – and remembered the explosions and showers of coloured light. What about the sulphurous smoke though? As atmospheric scientists have demonstrated, fireworks leave their mark on air quality for some time after the bangs and glows have passed.
After the annual Guy Fawkes Night in the UK, highly elevated levels of particles (smoke or soot) produced by the fireworks’ combustion, as well as high levels of metal ions such as magnesium that originate from the fireworks themselves, have been found. Firework displays have also been linked to elevated levels of other molecules such as nitrogen dioxide (NO2) and sulphur dioxide (SO2). Such observations were made during and after a Diwali festival in Hisar City, India, in November 1999; in Mainz, Germany, during New Year celebrations in 2004/2005; during the Lantern Festival in Beijing, China, in 2006; and in Milan, Italy, the night after Italy won the football World Cup in 2006 (Drewnick et al., 2006; Ravindra et al. 2003; Vecchi et al., 2008; Wang et al., 2007).

Together with your students, you too can analyse the effect of fireworks on air quality. We worked with UK secondary-school students to investigate the impact of Guy Fawkes Night on air quality (see acknowledgements). The project was an introduction to using air-quality databases – which contain measurements of a wide range of pollutants, a treasure trove of data for use in schools – but also a chance to carry out some real research at school.
Air quality can be linked to many school subjects. The chemistry and physics of fireworks involve a number of interesting topics, such as combustion, sound, light and the pollutants they can release. It can also form the basis of a deeper discussion of the nature of air pollution; what causes it, and effects such as acid rain and climate change. The latter are topics covered in biology, health and geography lessons. The analysis of data has huge potential for enlivening mathematics and IT lessons.
Information about the main pollutants caused by fireworks, as well as details of the chemistry of fireworks, can be downloaded from the Science in School websitew1. Further details on more general causes of air pollution can be downloaded from the UK-AIR website#w2.

You will need to use a publicly available air-quality database that provides at least daily measurements for the location you are interested in studying. The UK air-quality archivew2 contains hourly data for a range of chemical species; primary pollutants (emitted directly), including NO, NO2, CO and SO2; hydrocarbons and particulate matter; and secondary pollutants (formed from primary pollutants), such as ozone. The data are collected from 186 sites around the UK ranging from monitors at the roadside to those in remote regions for measuring background levels. Some sites have been working since the mid-1970s, providing an incredible record of data. The authors are keen to work with any groups of students who wish to interpret aspects of the UK’s air-quality data.
For Malta, there is the database of the Malta Environment and Planning Authorityw3 that contains data on CO, NO, NO2 and O3.
If you want to analyse data from another European country, you will find AirBasew4, the air-quality database maintained by the European Environment Agency, a useful resource as it contains measurements for most European countries. Note, however, that the files are large so can take some time to download, and are also less simple to understand than the UK and Maltese data sets.

We analysed particulate matter (PM) levels at all sites where they are measured in the UK around Guy Fawkes Night 2009. PM consists of particles of solid or liquid suspended in a gas. They are categorised according to size as PM10 (diameter 10 µm or less), PM2.5 (2.5 µm or less), PM1 (1 µm or less) and ultrafine (0.1 µm or less). Firework combustion produces a range of particle sizes but mainly smaller particles (e.g. PM2.5) of soot, whereas bonfires can form larger particles. PM is also produced by the construction industry, and there are natural sources such as pollen, sea salt and wind-blown soil. Increased levels of particles in the air are linked to cardiovascular and respiratory diseases; smaller particles are particularly unhealthy because they can penetrate deeper into the respiratory system. PM also has a significant effect on the climate: soot particles warm the climate, whereas reflecting articles tend to cool it.
As an example (Figure 1), we show PM2.5 and PM10 levels from the centre of Reading, a university town in the south of the UK. Although Guy Fawkes Night is actually on 5 November, it is frequently celebrated on the nearest weekend. These data from 5-9 November 2009 show that particle levels peaked on the evening of 7 November (a Saturday). Comparing those data to the all-year average for 2009, we found that the levels on that Saturday were elevated by a factor of up to seven (see Figure 1).
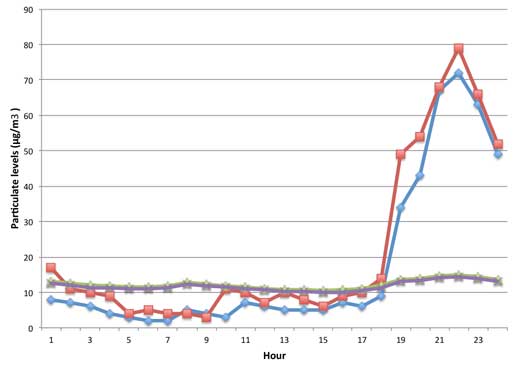
Because PM2.5 measures all particles with a diameter of 2.5 μm or less and the PM10 and PM2.5 levels are virtually the same, most particles produced were small – and particularly bad for the respiratory system. It is very difficult to set safe levels for particle exposure, but at present the limit for PM10 in Europe is an annual concentration of 40 μg/m3, and a daily concentration of 50 μg/m3, which must not be exceeded more than 35 times per calendar year (therefore called the exceedance). The average from the night of 7 November was 34.7 μg/m3, which is less than the exceedance, but much higher than the 2009 average (mean). At other sites in the UK, we found the PM10 level to be exceeded on that day.

These databases offer a wealth of possible questions to be considered at school, with examples by no means restricted to firework-derived pollution. For example:
The authors would like to acknowledge the help of the following teachers and students who participated in their UK air-quality study: Dr Oznur Kemal (teacher), Sophie Danby, Marta Tondera, Kelly Lam Ho, Candice Chan Ting Yan, Boni Chau Bo, Jenny Chow Kar Yee, Christine Fong Chi, Sophie Hawkins, Charlotte Hooper, Annabelle Fricker, Siobhan Stewart and Emma Tremewan, from Leweston School Dorset; Naomi Shallcross, Beth Shallcross and Esther Shallcross from Gordano School, Portishead; John Jones (teacher), Beth Jones and Cat Wood from Cheltenham College, Cheltenham.
All that glitters is certainly not gold. The popular practice of letting off spectacular fireworks, following a football victory or as a full-blown festival spread over several nights, may in fact be contributing to air pollution. This article provides references and ideas for teachers to approach the topic of air pollution by using specific occasions when high levels of gaseous and particulate pollutants are expelled into the air during firework displays. Science students have the opportunity for real-life scientific investigations; rather than confining their experiments to the school laboratory, they are immersed in the scientific community, working to make our environment a better place to live in.
The article could be used in several science subjects: for example, in chemistry (properties and reactions of metallic compounds; burning; stability of compounds; oxidising agents), physics (propulsion; light and sound), or biology or health lessons (the effects of pollution on respiratory diseases, especially asthma). It could also be used interdisciplinarily to consider global warming.
If the school can borrow the necessary equipment – for example, from a university – the school students could even take their own measurements of air quality, which would expose them to the technical analysis of the air, data logging and handling, data comparison and error analysis. They could then perhaps present their findings to the local authority, experiencing for themselves how reliable results of scientific investigations can be used to put pressure on policy makers and potentially bring about improvements.
Angela Charles, Malta