Virtual labs, real science Inspire article
Not just for remote teaching: virtual labs really came into their own during the COVID-19 pandemic, but they can generally be a useful addition to the STEM teacher’s toolbox.
Introduction
Textbook content and practical experiments are both critical for science education. Experiments and demonstrations help to engage students’ attention and can additionally help them to understand the concepts learned.
With many teachers having to teach remotely during the COVID-19 pandemic, practical experiments have become difficult. One option to introduce a more practical element into online lessons is the use of virtual labs. As the name suggests, a virtual laboratory is a computer-based activity where students interact with simulated computer interfaces to perform experiments using virtual tools, apparatus, or materials.[1,2]
However, many teachers are hesitant to try virtual labs due to concerns that they might be expensive or complicated, or that it isn’t worth setting everything up to use them when they won’t be needed after the pandemic. In fact, many of these tools are easy to use and some, like PhET Interactive Simulations (PhET), are available for free. Furthermore, while virtual labs can’t replace the experience of doing physical experiments, they have features that are complementary to practical classes and be a useful supplement even during normal teaching.[3] For example, the virtual models in these programs allow students to visualize processes, like molecular motion, that would normally be invisible.
This article introduces PhET – its basic features and some of the simulations it offers – in the hope of inspiring teachers to give these systems a try. A comparison with some other simulation platforms is provided at the end.
PhET Interactive Simulations
PhET is one of the most popular simulation platforms, is available for free, and provides interactive simulations for a range of scientific topics.[4,5] It was launched in 2002 by a group of physicists at the University of Colorado Boulder (USA), led by Nobel laureate Dr Carl Wieman.[6]
PhET is not technically difficult to use. Teachers can either download the simulations or use them online, and instructions are provided under ‘Teacher Tips’ on the homepage for each simulation. All simulations are accompanied by explanations of the basic theory, handouts (including tips for teachers), activity worksheets, and discussion questions. Teachers can add their own set of questions or update the existing set to check the conceptual understanding of the students.
PhET stands for Physics Education Technology and initially offered only physics-based simulations. Since then, it has been expanded to simulations involving chemistry, biology, mathematics, and earth science (figure 1). PhET simulations are designed to engage students through an intuitive, story-telling or game-like environment, where students learn through exploration. Currently, PhET has a total of 159 interactive simulations for primary and secondary school students across five disciplines.
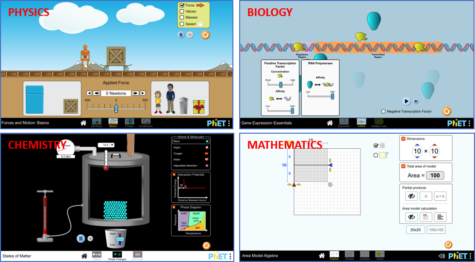
Image: PhET Interactive Simulations/ University of Colorado Boulder
Examples of PhET simulations
States of matter is a fundamental topic in chemistry and is critical for students’ conceptual understanding before moving on to more complex concepts. Unfortunately, students often have difficulty visualizing the fundamental particles (atoms and molecules) and their motion. PhET provides an opportunity not only to visualize the organization of the atoms and molecules in a particular state, but also to see the changes that take place during changes of state (figure 2).
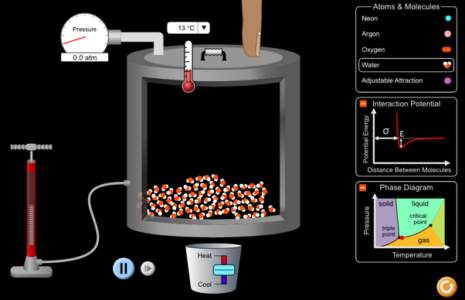
Image: PhET Interactive Simulations/ University of Colorado Boulder
From the simulation screen, users can select different molecules from a list (neon, argon, oxygen, and water). They can then change the temperature and pressure of the system and see how the arrangement and behaviour of the particles change (figure 3).
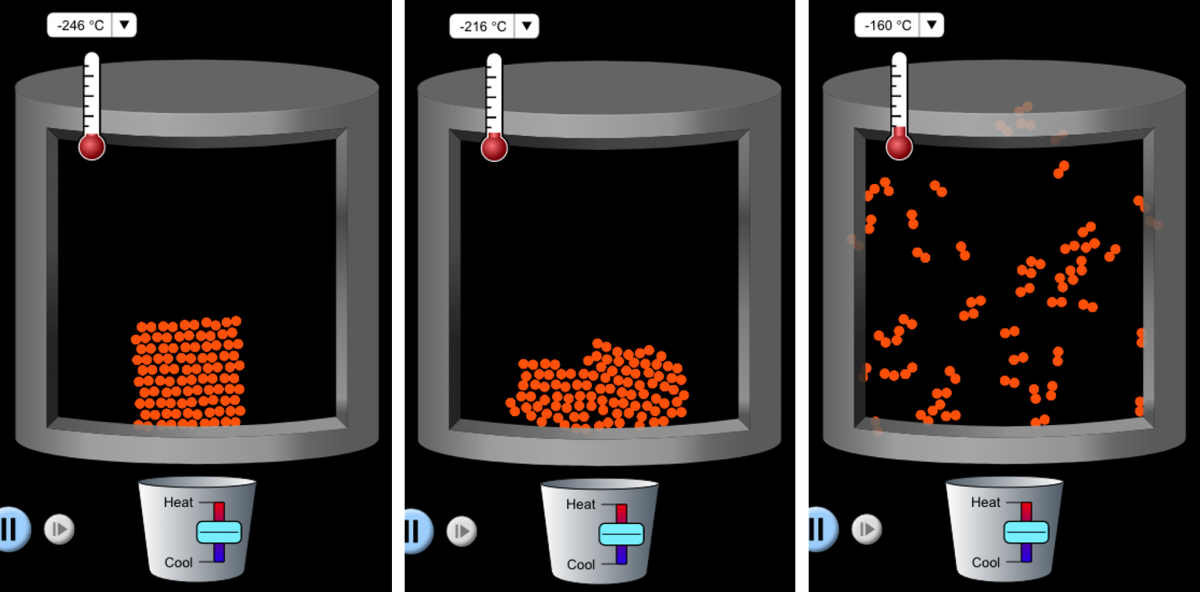
Image: PhET Interactive Simulations/ University of Colorado Boulder
This is particularly useful for explaining why ice floats on water, which often confuses students based on their prior knowledge that solids are denser than the corresponding liquid. With this simulation, students can easily visualize how water molecules assemble into a cage-like structure when the temperature is lowered, thus making ice less dense than liquid water (figure 4).
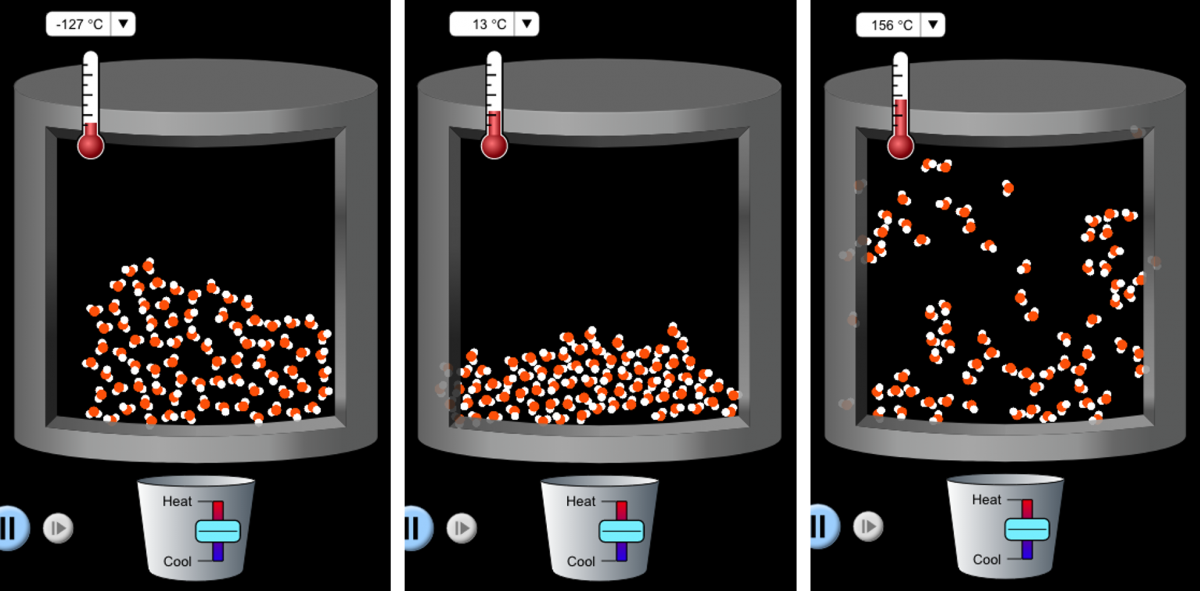
Image: PhET Interactive Simulations/ University of Colorado Boulder
There is also a range of simulations relating to electricity, covering topics such as electrical charge and conductivity, electrostatics, resistance and conductance, and circuit construction. An example is ‘Electric Field Hockey’, in which students can learn about electrical charges and their interaction by trying to score goals in a simulated ice-hockey game, and there are several other fun activities presented in a game format that students can explore themselves (figure 5).
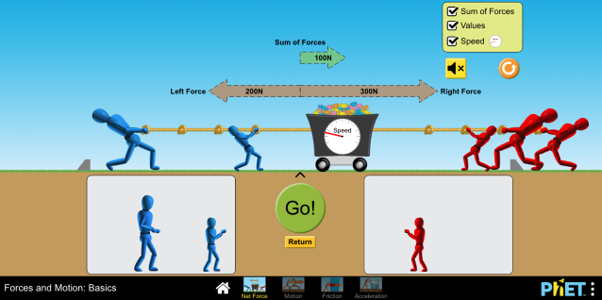
Image: PhET Interactive Simulations/ University of Colorado Boulder
PhET can also be useful to demonstrate the phenomena that influence climate change and global warming. The greenhouse effect simulation, for example, demonstrates how the earth absorbs sunlight and re-emits it as infrared radiation, and how clouds and greenhouse gases prevent this radiation from escaping into space (figure 6). In the photon absorption tab, students can choose different atmospheric molecules and see how they interact with photons of sunlight or infrared radiation.
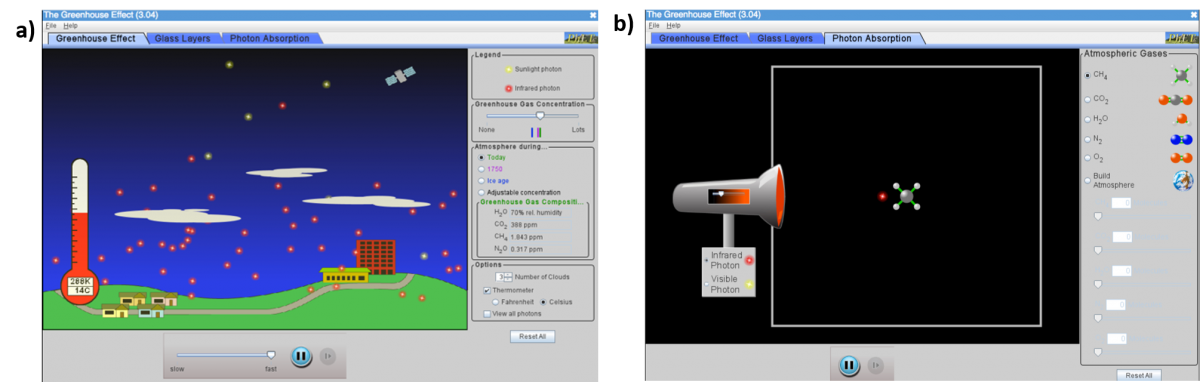
Image: PhET Interactive Simulations/ University of Colorado Boulder
Other virtual labs
PhET is a pioneer in virtual simulations and remains relevant and heavily accessed even after almost two decades. Unfortunately, PhET currently offers relatively few simulations for biology, mathematics, and earth science, although new simulations are continually being added. Furthermore, the interface may appear slightly outdated compared to some of the commercially available virtual labs, and some of the simulations may be difficult for the students to complete without guidance from their teachers. It would also be useful if PhET were to offer a proper assessment option.
There are several other virtual labs, either commercially available or open-source, that offer an alternative. Table 1 provides a comparison of four of these (PhET, OLabs, Beyond Labz, and Labster), chosen based on their features and applicability for a wide range of subjects for school education, integration with different institutions, and a large number of users [4, 7–9]. There are several other virtual labs that offer mostly subject-specific simulations, and a comprehensive list can be found in Ref. [10].
| Phet | OLabs | Beyond Labz | Labster | |
|---|---|---|---|---|
| Topics | physics, chemistry, biology, mathematics, earth science | physics, chemistry, biology, maths | general chemistry, organic chemistry, biology, physics, physical science | physics, chemistry, biology, medicine, engineering |
| Target audience | Primary school to secondary school | middle school to secondary school | middle school upwards | high school upwards |
| Platform | website, app | website | website | website, app (beta version available only in selected countries) |
| Connectivity | online/offline | online | online/ offline | online |
| Access | open-source/free | open-source/free | free trial period; then paid access | free trial period, free lab safety simulation; then paid access |
More realistic and sophisticated virtual labs are usually behind a paywall, which can be a hurdle for access unless the educational institution can pay for it.
Summary
Virtual labs have been slowly gaining momentum for the past few years, and many research groups, start-ups, and big companies have been working on improving existing simulations or developing new ideas. However, with widespread remote teaching during the COVID-19 pandemic, these tools really came into their own, and they have been extensively used to support online STEM teaching. PhET, for examples, saw a significant increase in its usage worldwide, and up to a 500% increase in countries like France and Italy.[11] These tools are useful beyond remote teaching though, and can be a valuable supplement to practical classroom experiments.
References
[1] Woodfield et al. (2004) The Virtual ChemLab Project: A Realistic and Sophisticated Simulation of Inorganic Qualitative Analysis. J. Chem. Educ. 81:1672–1678. doi:10.1021/ed081p1672
[2] Rutten N, van Joolingen WR, van der Veena JT (2012) The learning effects of computer simulations in science education. Computers & Education 58:136–153. doi:10.1016/j.compedu.2011.07.017
[3] Ali N, Ullah S Review to Analyze and Compare Virtual Chemistry Laboratories for Their Use in Education. J. Chem. Educ. 97:3563–3574. doi:10.1021/acs.jchemed.0c00185
[4] PhET: https://phet.colorado.edu/
[5] Moore EB et al. (2014) PhET Interactive Simulations: Transformative Tools for Teaching Chemistry. Journal of Chemical Education 91:1191–1197. doi: 10.1021/ed4005084
[6] Finkelstein N et al. (2006) High tech tools for teaching physics: The physics education technology project. MERLOT Journal of Online Learning and Teaching 2:110–121.
[7] OLabs: http://www.olabs.edu.in/
[8] Beyond Labz: https://www.beyondlabz.com/
[9] Labster: https://www.labster.com/
[10] Open Educational Resources: Simulations and Virtual Labs, https://libguides.mines.edu/oer/simulationslabs
[11] Leytham-Powell C (2020) PhET simulations keep students engaged while learning science remotely. Colorado Arts and Science Magazine
Resources
- PhET interactive simulations: https://phet.colorado.edu/
- A collection of Science in School articles ideal for remote teaching: Watt S (2020) Science at home: ideas for remote teaching. Science in School 50
- Some remote learning ideas from EMBL: Dobreva T, Haas E (2020) Science at home: distance learning with EMBL. Science in School 50
- Other online resources for students: Prior E (2020) Keeping science engaging: Online resources for students during COVID-19. Science in School 50





