Starch: a structural mystery Understand article
A string of glucose molecules: starch. It sounds simple, but it isn’t. Dominique Cornuéjols and Serge Pérez explore the intricacies of its structure – and show that the mystery is by no means solved.

Klement / iStockphoto
Take a handful of spaghetti and throw it into boiling water. The stiff sticks will quickly become softer and gently curve while swelling. We take this behaviour of noodles in hot water for granted, but what exactly is happening to the fine structure of spaghetti to result in such a dramatic metamorphosis?
The beginning of the answer is that pasta – like rice, potatoes or bread – contains a large amount of starch. But what is starch? Produced in plants by the photosynthesis of carbon dioxide, starch granules are made out of glucose polymers and serve as energy stores. Towards the end of the growing season, starch accumulates in twigs of trees, close to the buds. It is also found in fruits, seeds, rhizomes and tubers. Starch granules are very suitable for such long-term storage, because of their compactness, relative dryness and high stability.
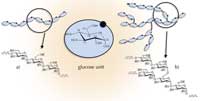
motifs of amylose (a) and
amylopectin (b). Click to
enlarge image
Image courtesy of Serge Pérez,
ESRF
However, this essential source of energy only became accessible to humans once they had tamed fire, because raw starch granules are so compact that they are hardly digestible. In order to increase its digestibility, starch needs to be cooked: it is only once it has been heated that it becomes water-soluble and edible.
The transformation of raw starch in hot water is called gelatinisation: the granules swell and burst, forming a paste. During cooling or prolonged storage, the starch paste often thickens due to a phenomenon called retrogradation. Gelatinisation and retrogradation, which correspond to structural modifications in the granules, affect the behaviour of starch-containing systems.
Consequently, starch is excellent for modifying the texture of many processed and home-cooked foods (for example, as flour or corn flour to thicken sauces), and has also been used for centuries for other purposes, including the manufacture of paper (sizing), glues or fabric stiffener. Today, new applications of starch are emerging, including low-calorie dietary fibres, biodegradable packaging materials, thin films and thermoplastic materials.
Science: one small step at a time
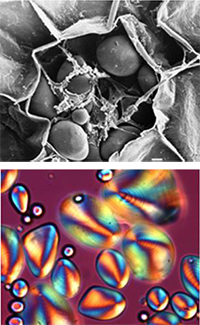
granule observed by scanning
electron microscopy b) The
corresponding granule under
polarised light
Image courtesy of Serge Pérez,
ESRF
Starch, therefore, is widely used in industry – and has been for thousands of years. The scientific study of starch started in 1833 when the French chemist Anselme Payen identified starch as being composed of glucose units. However, even today, its biochemistry and detailed structure are not yet well understood. At a molecular level, we know that native starch (as it occurs naturally) is made of two distinct components, amylose and amylopectin, which can be isolated by fractionation and studied independently.
Both components contain polymer chains of glucose units, but the chains are linked differently. Amylose is mainly linear (with glucose units linked by (1-4) bonds (Figure 1a), whereas amylopectin has a highly branched, very dense structure, due to (1-6) linkage (Figure 1b). Amylopectin can contain up to a hundred thousand glucose residues and is the largest known biomacromolecule.
Native starch granules vary enormously in shape and size (from 0.1 to 200 mm), but they all have a common characteristic: under the microscope and illuminated with polarised light, starch grains stained with iodine exhibit a distinctive ‘Maltese cross’ (shown in orange in Figure 2b), indicating the existence of some common internal ordering. When granules are heated in excess water (as when spaghetti is cooked), the polarisation cross begins to disappear, demonstrating that this molecular order is being disrupted.
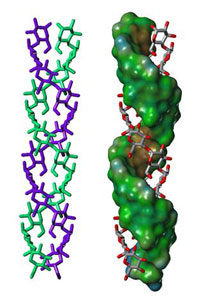
structure in amylopectin
Image courtesy of Serge Pérez,
ESRF
The physical properties of starch – its stability and phase transformations, for example from starch granules to gels, or from brittle, raw pasta to soft, cooked pasta – are directly linked to this molecular order. However, understanding the detailed structure of starch requires very advanced research tools and techniques, such as X-ray crystallography, electron microscopy, nuclear magnetic resonance and computer modelling.
With the help of these tools, scientists are slowly beginning to build up a picture of how starch – something we are so familiar with – is constructed.
Starting from the nanoscale: double helices, lamellae and superhelices
X-ray diffraction investigations at the nanoscopic level indicate that:
- Starch is composed of thin lamellar domains (about 4.5 nm thick);
- Each lamella is made up of about 100 double-stranded helices, each consisting of about 20 glucose units (Figure 3);
- The double helices are very densely packed, with a high degree of regularity, like in a crystal (see glossary for all italicised terms).
(For an explanation of how X-ray diffraction is used to analyse crystalline structures, see Cornuéjols, 2009.)
b) Possible entanglement between an amylose chain (red) and amylopectin double helices (green and yellow)
Image courtesy of Serge Pérez, ESRF

Possible model
of a superhelix
structure
Image courtesy
of Serge Pérez,
ESRF
These X-ray results corroborate biochemical studies of the amylopectin molecule which show that this huge molecule is organised in crystalline clusters of double helices (Figure 4a): the lamellae revealed in the X-ray diffraction research consist of the clusters of double helices demonstrated in the biochemical studies. In this model, the branch points (the (1-6) links) in the amylopectin molecules are located in the less organised (or more amorphous) regions between the clusters. Amylose is entangled with amylopectin (Figure 4b), but so far nobody knows exactly how.
Additional X-ray studies, using the techniques of small-angle and wide-angle scattering (SAXS and WAXS) to analyse hydrated starch, show that the lamellae of double helices are probably organised in a helical superstructure, or superhelix (Figure 5). (For an explanation of SAXS, see Stanley, 2009.)
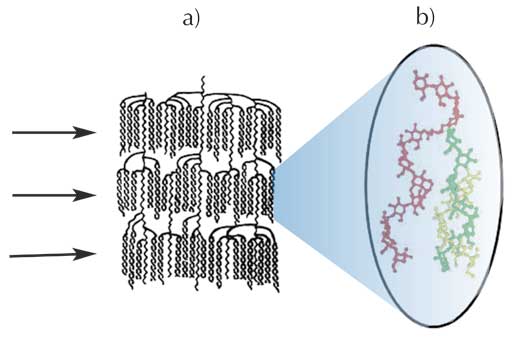
Down from the microscale: growth rings and blocklets

consisting of alternate
layers of amorphous and
semi-crystalline regions.
Click to enlarge image
Image courtesy of Serge
Pérez, ESRF
The lamellar and superhelix structures of amylopectin are only a small part of the whole picture, however. On a larger (microscopic) scale, it is known that starch granules are made up of alternating amorphous and semi-crystalline shells, between 100 and 800 nm thick. These structures are termed growth rings (Figure 6).
We know almost nothing about the amorphous parts of the growth rings. The more crystalline regions, however, can be studied by X-ray diffraction. Recently experiments using extremely focused X-ray beams at a synchrotron (see box) have demonstrated that within the semi-crystalline regions, the nanoscopic lamellae are parallel to the surface of the starch granule (Figure 7). This enabled scientists to make the link between the small (microscale, e.g. the starch granules) and the very, very small (nanoscale, e.g. lamellae) – a link that is difficult to obtain in such structural investigations.
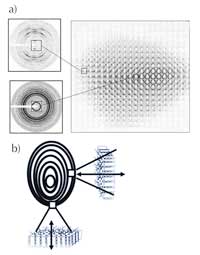
diffraction images taken at
different places in the
granule (‘mapping’ of the
granule) b) Orientation of
the lamellae vis-à-vis the
surface of the granule.
Click to enlarge image
Image courtesy of Serge
Pérez, ESRF
Another recent study, using atomic force microscopy to look at the starch granule’s surface, showed the presence of blocklets within the growth rings. These blocklets are more or less spherical and have a size of 20–100 nm. So far, however, nothing more is known about these blocklets.
Taking all the studies together, we can be fairly sure about nanoscale structure (double helices forming lamellae) and the growth rings (alternating amorphous and semi-crystalline shells); however, the evidence for the intermediate structures (the superhelices and the blocklets) is less solid. Furthermore, it is still unclear how the superhelices, blocklets and growth rings relate to each other. Figure 8 summarises the different structural levels (glucose units, helices, lamellae, superhelices, blocklets and growth rings), from the molecular (10-9 m) to the microscopic (10-5 m) level.
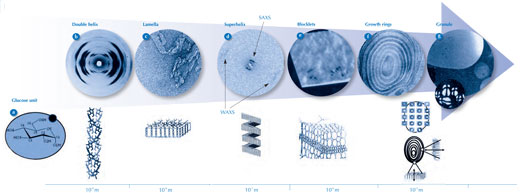
a) Glucose unit
b) Double helix
Top: X-ray fibre diffraction pattern demonstrating a double-helix structure (courtesy of Imberty et al., 1988)
Bottom: model of the double-helix structure
c) Lamella
Top: transmission electron microscopy image of hydrolysed starch, showing the shape of the crystalline lamellae (courtesy of Angellier-Coussy et al., 2009)
Bottom: model of a crystalline lamella made of about 100 double helices
d) Superhelix
Top: small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS) diffraction images indicating the presence of a superhelix structure (courtesy of Waigh et al., 2000)
Bottom: the superhelix model, with a pitch of 9 nm and a diameter of 18 nm
e) Blocklets
Top: atomic force microscopy image of the typical surface of a starch granule (courtesy of Gallant et al., 1997). The bumps seen on the surface indicate the presence of blocklets
Bottom: blocklet model. The blocklets are believed to be smaller in the amorphous regions (central region) than in the semi-crystalline regions (above and below)
f) Growth rings
Transmission electron microscopy image of an ultrathin section of hydrolysed starch granule, showing the growth rings as alternate layers of amorphous and semi-crystalline regions (courtesy of I. Paintrand, CERMAV, Grenoble, France)
g) Granule
Top: starch granule observed by scanning electron microscopy (large image) and the corresponding granule under polarised light (inset)
Middle: set of microfocus X-ray diffraction patterns recorded on a starch granule showing the distribution and orientation of the crystalline domains in a starch granule. Each diffraction pattern corresponds to an area of about 3 μm2 of the specimen and steps of 7 μm separate two patterns (courtesy of Buleon et al., 2009)
Bottom: starch granule section showing the radial orientation of the crystalline domains (lamellae) in a starch granule
Click on image to enlarge
Image courtesy of Serge Pérez, ESRF
As early as 1858, the Swiss botanist Carl von Nägeli had a brilliant intuition, stating that “The starch grain… opens the door to the establishment of a new discipline… the molecular mechanics of organised bodies.” He would no doubt be astonished that, more than 150 years later, we are still struggling to understand the complex architecture of starch granules.
Glossary
Amorphous: Describes a material (or part of material) that has no organisation and no order.
Crystal: A perfect crystal is a solid material whose constituent atoms, molecules or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions.
Crystalline: Having the properties of a crystal; by extension, characterises parts of a material that are ordered (for example, a cluster of double helices all packed with the same orientation of the helix axis).
Semi-crystalline: Describes a material (typically a biopolymer) with both amorphous and crystalline parts.
ESRF
The European Synchrotron Radiation Facility (ESRF)w1 in Grenoble, France, is a good example of a large facility operating day and night for the benefit of thousands of users from all over the world. A ‘user’ is a scientist, usually part of a larger team, who occasionally needs a powerful tool to obtain information on a sample of interest (a polymer, a protein crystal, a fossil or a catalytic reaction, for instance).
ESRF produces extremely intense X-rays, called synchrotron radiation. These X-ray beams are emitted by high-energy electrons, which circulate in a large storage ring, 844 m in circumference. The X-ray beams are directed towards the beamlines, which surround the storage ring in the experimental hall. Each of the 42 beamlines at the ESRF is specialised in a specific technique or type of research. For around half a dozen of them, this speciality is polymers.
In the future, polymer research will benefit from the newly created Partnership for Soft-Condensed Matter (which includes polymers). The introduction of nanobeams (even more focused, nano-sized X-ray beams) will soon allow even finer structural analysis and yet more progress in the study of polymers, including starch.
References
- Angellier-Coussy H, et al. (2009) The molecular structure of waxy maize starch nanocrystals. Carbohydrate Research 344: 1558-1566. doi: 10.1016/j.carres.2009.04.002
- Buléon A, Véronèse G, Putaux JL (2007) Self-association and crystallization of amylose. Australian Journal of Chemistry 60: 706-718. doi: 10.1071/CH07168
- Cornuéjols D (2009). Biological crystals: at the interface between physics, chemistry and biology. Science in School 11: 70-76. www.scienceinschool.org/2009/issue11/crystallography
- Gallant DJ, Bouchet B, Baldwin PM (1997) Microscopy of starch: evidence of a new level of granule organization. Carbohydrate Polymers 32: 177-191. doi: 10.1016/S0144-8617(97)00008-8
- Imberty A et al. (1988) The double-helical nature of the crystalline part of A-starch. Journal of Molecular Biology 201: 365-378. doi: 10.1016/0022-2836(88)90144-1
- Stanley H (2009) Plasma balls: creating the 4th state of matter with microwaves. Science in School 12: 24-29. www.scienceinschool.org/2009/issue12/fireballs
- Waigh TA et al. (2000) Side-chain liquid-crystalline model for starch. Starch 53: 450-460. doi: 10.1002/1521-379X(200012)52:12<450::AID-STAR450>3.0.CO;2-5
Web References
w1 – To learn more about ESRF, see: www.esrf.eu
Resources
- For an extensive review of starch, see ESRF scientists’ Serge Pérez and Anne Imberty’s ‘Starch: structure and morphology’ website: www.cermav.cnrs.fr/glyco3d/lessons/starch
- Imberty A, Pérez S (1988) A revisit to the three-dimensional structure of B-type starch. Biopolymers 27: 1205-1221. doi: 10.1002/bip.360270803
- Pérez S, Baldwin P, Gallant DJ (2009) Structural features of starch. In: Starch-Chemistry and Technology, 3rd edition. BeMiller J, Whistler R (eds.). pp149-192. New York, NY, USA: Academic Press. ISBN: 978-0127462752
- Chanzy H, et al. (2006) Morphological and structural aspects of the giant starch granules from Phajus grandifolius. Journal of Structural Biology 154(1): 100-120. doi:10.1016/j.jsb.2005.11.007
Institutions
Review
The ultra structure of starch is not often considered, but this article describes how the components of starch – amylose and amylopectin – form the complicated levels of structure within the polymer. The article could be used as extension work in a lesson on starch digestion or measurement. It could also be used as an example of the use of the technique of X-ray diffraction or polarising light microscopy. Posters could be made (or homework set) on the uses of starch in industry, with each group taking a different use. Methods for producing and / or assaying starch in industry could be investigated. Comprehension questions could be set; for example:
- Starch is made up of 2 components that are:
- Amylase and amylopectin
- Amylose and amylopectin
- Glucose and amylase
- Glucose and amylose
- Describe what gelatinisation is.
- What are growth rings in starch?
- What is the difference between amylose and amylase?
- Convert the sizes of the different structural levels from 10-9 m into smaller units, e.g. micrometres or nanometres.
- X-ray diffraction was used in the 1950s to determine the structure of a well known molecule. Which molecule?
Shelley Goodman, UK
