A neural switch for fear Understand article
When something frightens us, should we freeze, or should we investigate? Sarah Stanley describes how scientists from the European Molecular Biology Laboratory are probing the mysteries of the brain, seeking to understand our response to fear.

photolab
Flee, fight or freeze? For an animal overcome with fear, that is the essential question. The answer often depends on the amygdala – a major emotion-processing hub nestled deep in the brain. In both mice and humans, it affects how we behave in response to certain types of fear, and helps to form long-term memories of frightening experiences. However, very little is known about how cells in the amygdala communicate with other brain cells to produce specific fear-induced behaviours.
A recent study narrows this gap in knowledge, thanks to the innovative work of scientists at the European Molecular Biology Laboratoryw1 (EMBL) in Monterotondo, Italy, and GlaxoSmithKlinew2 in Verona, Italy. The scientists focused on one of many different types of fear processed by the amygdala. They used new techniques to understand the interactions between the areas of the brain involved in reactions to that specific type of fear. In the course of their work, they identified a switch that toggles between two different fear responses: freezing and, surprisingly, an alternative to the flee, fight or freeze options, known as active risk assessment. This active response involves behaviours like rearing, digging and exploring.
Mice conditioned to associate a tone with an uncomfortable shock usually freeze in fear upon hearing the sound, even if it is not accompanied by discomfort. Neurons called type 1 cells, which are found in the amygdala, are known to control the freezing response. When type 1 cells are prevented from sending signals to other cells, mice no longer freeze in fear. But, it seems, type 1 neurons are more than just an on / off switch.
In a pioneering approach that uses both pharmacology and genetics, EMBL scientists engineered mice so that their type 1 cells could be turned off without disrupting other cells. The mice were made to produce certain drug-sensitive proteins (receptors) exclusively in their type 1 cells. When the mice were injected with the drug, it bound to those receptors, setting off chemical reactions that disrupted the cells’ electrical charge. Thus, these neurons could no longer send electrical signals to surrounding brain regions.

Image courtesy of EMBL
Photolab
Before treatment with the drug, the mice had been conditioned to fear a certain tone. After their type 1 cells were blocked, they were subjected to the tone, and their behaviours were observed and analysed.
“When we inhibited these neurons, I was not surprised to see that the mice stopped freezing, because [freezing] is what the amygdala was thought to [control]. But we were very surprised when they did a lot of other things instead, like rearing and other risk-assessment behaviours,” says Cornelius Gross, who led the research at EMBL. “It seemed that we were not blocking the fear, but just changing their responses from a passive to an active coping strategy. That is not at all what this part of the amygdala was thought to do.”
To better understand the connections between brain cells – the neural circuitry – involved in the switch from passive to active fear behaviours, the scientists looked at activity in different brain regions using a type of brain scan called functional magnetic resonance imaging (fMRI). In small animals like mice, fMRI measures local blood volume as an indicator of brain activity: the more blood there is in a particular area of the brain, the more active those neurons are. This study marks the first use of fMRI to map neural circuitry in mice, using a new technique developed by GlaxoSmithKline scientist Angelo Bifone and his team.

Photolab
The brain scan yielded another unexpected result. Scientists previously thought that the amygdala managed fear behaviours by simply relaying information to the brainstem, which links the brain to the spinal cord. But Cornelius, Angelo and their colleagues found that in mice with blocked type 1 cells, the outer layer of the brain – the cortex – was highly active, indicating that it too plays a role in determining how mice react to fear. Activity was also observed in a region of the brain called the cholinergic basal forebrain, which is known to influence cortex activity.
Like all brain scans, fMRI requires the subject to remain very still, so it can only be performed on mice that have been anaesthetised. But the scientists wanted to confirm the association between the cortex and fear behaviours in conscious mice. As they could not observe brain activity while the mice were awake and therefore capable of displaying fear behaviour, the scientists took a different approach. They used the drug atropine to block cortex activity in mice whose type 1 cells were blocked, and found that the animals no longer showed any risk assessment behaviours.
Thus, the scientists infer, the amygdala normally inhibits the cholinergic basal forebrain, while it signals the brainstem to control the passive fear response: freezing (see image below, A). When type 1 neurons are blocked, however, the amygdala releases its hold on the cholinergic basal forebrain, leading to cortex activity and an active reaction to fear: risk assessment (see image below, B).
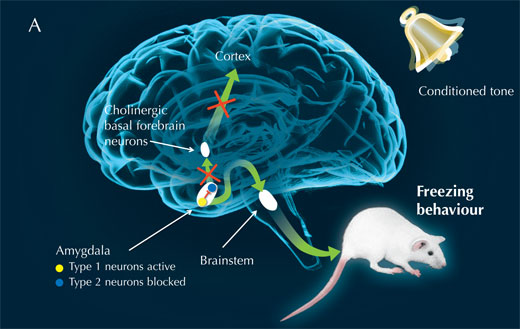
Image courtesy of Nicola Graf, Cornelius Gross and Marlene Rau
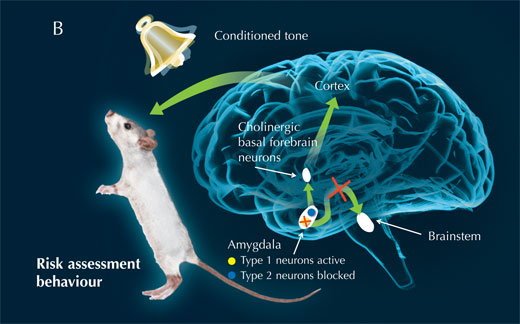
Image courtesy of Nicola Graf, Cornelius Gross and Marlene Rau
“This is a powerful demonstration of the ability of functional MRI to resolve brain circuits involved in complex tasks, like processing of emotions and control of behavioural responses,” says Angelo, now at the Italian Institute of Technologyw3 in Pisa.
Taken together, the results of the array of techniques used to explore the freezing fear response in mice indicate that the amygdala plays a more complex role in fear processing than previously thought. Instead of merely passing on information about external threats, the amygdala makes decisions about how to respond.
It is important to note that the type of fear explored in this study – conditioned fear of a painful shock – is very specific. The results cannot necessarily be applied to behavioural responses to other types of fear in mice.
“There are multiple, parallel fear circuits that handle different types of fear information. For example, one part of the [mouse] brain is often used to process fear of a predator, such as a cat, while another part usually responds to aggressive behaviour by another mouse,” Cornelius says. “We thought there was a simplistic fear circuit that is either on or off, but that doesn’t seem to be true.”
Also, scientists are not yet sure whether wild mice ever use risk assessment behaviours in response to threatening stimuli. Blocking of type 1 cells was performed artificially in this study, and there may or may not be situations when the neurons would naturally be inhibited, leading the mouse to engage in investigative behaviours to learn more about a perceived threat.
If the active response is found naturally in mice, what kinds of external sensory cues are necessary to activate it? Previous studies have shown that animals located farther away from a perceived threat are more likely to freeze in fear than run or fight. But scientists cannot yet say whether use of an active risk assessment response is a function of distance. Cornelius emphasises that it is important not to assume that risk assessment would be used in place of freezing in a situation perceived as less threatening.
Nonetheless, the study has significant implications. The pharmacogenetic and fMRI techniques used by the scientists will likely prove invaluable in many other studies of neural circuits in mice. Indeed, Cornelius and his team have already used a pharmacogenetic approach to reveal cells that act as an input for another brain region, the hippocampus, relaying information that enables a mouse to gauge an appropriate level of anxiety in an uncomfortable circumstance.
Furthermore, we humans also show freezing and risk assessment responses to fear. We possess a region of the amygdala that is analogous to the one housing the active / passive switch in mice. Patients who have suffered lesions to this region are unable to develop conditioned fear responses, though they have normal reactions to fear in other situations. Thus, it is likely that the results of this study could apply directly to humans, Cornelius says.
Though much remains to be discovered about how humans process fear in different situations, studying fear brings scientists ever closer to developing more effective treatments for fear-based illnesses – such as anxiety disorders and post-traumatic stress disorder. In the words of two-time Nobel Prize-winning chemist Marie Curie, “Now is the time to understand more, so that we may fear less.”
References
- Gozzi et al. (2010) A neural switch for active and passive fear. Neuron 67(4): 656-66. doi: 10.1016/j.neuron.2010.07.008
Web References
- w1 – To learn more about the European Molecular Biology Laboratory (EMBL), see: www.embl.org
- w2 – For information about GlaxoSmithKline in Verona, Italy, see: www.gsk.it
- w3 – To find out more about the Italian Institute of Technology, see: www.iit.it
Resources
- For an introduction to the 2010 article by Gozzi et al., see:
- Pape HC (2010) Petrified or aroused with fear: the central amygdala takes the lead. Neuron 67(4): 527-529. doi: 10.1016/j.neuron.2010.08.009
- As part of an exhibition (Goose Bumps! The Science of Fear), the Museum of Science, Boston, USA, devised some scary activities for the classroom. See the museusm’s website (www.mos.org) or use the direct link: http://tinyurl.com/65savzz
- For another example of research using fMRI, see:
- Hayes E (2010) The science of humour: Allan Reiss. Science in School 17: 8-10.
Institutions
Review
In this article, several experiments are carried out with mice (in which both the behaviour and the activity of the brain were analysed) to understand the details of their response to fear. Such research is very important to ultimately improve our knowledge about how the human brain works.
This article can be extremely useful for giving students some insight into how research is done in scientific laboratories. Teachers can get their students to read the article and then think about how they respond to fear, perhaps even designing and performing an experiment. Furthermore, students can think about the evolutionary benefits of these reactions for our ancestors, and how useful they are in modern life. Moreover, the students can try to find out about animals with different responses to fear and try to relate their behaviour to their environment.
The use of new research techniques such as pharmacogenetics and functional magnetic resonance imaging (fMRI) is also reported in this article. Students aged 16 and over can try to find more information about how these techniques work and their importance in research.
Finally, this article could also be used as a starting point for discussing research on animals. Students can think about how the results obtained from animals can be applied to humans, and they can also discuss alternatives to animal testing.
Mireia Guell Serra, Spain
